Taxonomy and Molecular Phylogeny of Phellodon (Thelephorales) with Descriptions of Four New Species from Southwest China
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation of Ectomycorrhizal Fungi: Exploring the Linkages Between Functional and Taxonomic Responses to Anthropogenic N Deposition
fungal ecology 4 (2011) 174e183 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/funeco Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition E.A. LILLESKOVa,*, E.A. HOBBIEb, T.R. HORTONc aUSDA Forest Service, Northern Research Station, Forestry Sciences Laboratory, Houghton, MI 49931, USA bComplex Systems Research Center, University of New Hampshire, Durham, NH 03833, USA cState University of New York, College of Environmental Science and Forestry, Department of Environmental and Forest Biology, 246 Illick Hall, 1 Forestry Drive, Syracuse, NY 13210, USA article info abstract Article history: Anthropogenic nitrogen (N) deposition alters ectomycorrhizal fungal communities, but the Received 12 April 2010 effect on functional diversity is not clear. In this review we explore whether fungi that Revision received 9 August 2010 respond differently to N deposition also differ in functional traits, including organic N use, Accepted 22 September 2010 hydrophobicity and exploration type (extent and pattern of extraradical hyphae). Corti- Available online 14 January 2011 narius, Tricholoma, Piloderma, and Suillus had the strongest evidence of consistent negative Corresponding editor: Anne Pringle effects of N deposition. Cortinarius, Tricholoma and Piloderma display consistent protein use and produce medium-distance fringe exploration types with hydrophobic mycorrhizas and Keywords: rhizomorphs. Genera that produce long-distance exploration types (mostly Boletales) and Conservation biology contact short-distance exploration types (e.g., Russulaceae, Thelephoraceae, some athe- Ectomycorrhizal fungi lioid genera) vary in sensitivity to N deposition. Members of Bankeraceae have declined in Exploration types Europe but their enzymatic activity and belowground occurrence are largely unknown. -
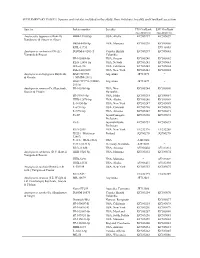
Suppl Table I
SUPPLEMENTARY TABLE I. Species and isolates included in the study, their vouchers, locality and GenBank accession Species Isolate number Locality ITS GenBank LSU GenBank accession no. accession no. Amylocystis lapponica (Romell) HHB-13400-Sp USA, Alaska KC585237 KC585059 Bondartsev & Singer ex Singer OKM-4418-Sp USA, Montana KC585238 KC585060 KHL-11755 − EU118603 Amyloporia carbonica (Overh.) DAOM-F-8281-T Canada, British KC585239 KC585061 Vampola & Pouzar Columbia FP-105585-Sp USA, Oregon KC585240 KC585062 RLG-12496-Sp USA, Nevada KC585241 KC585063 Wilcox-96 USA, California KC585242 KC585064 Zabel-40-GLN USA, New York KC585243 KC585065 Amyloporia nothofaginea Rajchenb. BAFC519794 Argentina JF713078 − & Gorjón (=MMBP-2011) BAFC519796 (=MBP- Argentina JF713079 − 2011a) Amyloporia sinuosa (Fr.) Rajchenb., FP-105386-Sp USA, New KC585244 KC585066 Gorjón & Pildain Hampshire FP-94464-Sp USA, Idaho KC585245 KC585067 HHB-12878-Sp USA, Alaska KC585246 KC585068 L-14130-Sp USA, New York KC585247 KC585069 L-6192-Sp USA, Colorado KC585248 KC585070 L-9792-Sp USA, Arizona KC585249 KC585071 Pa-3C JapanYamagata KC585250 KC585072 Prefecture Pa-3e JapanShizuoka KC585251 KC585073 Prefecture RLG-2538 USA, New York EU232196 EU232288 X725 (=Miettinen- Finland JQ700270 JQ700270 12407) P-115 (=RLG-2538) USA AJ416068 − P-211 (G-214) Germany, Karlsrube AJ345011 − RLG-1182R USA, Arizona AY966450 AY333831 Amyloporia sitchensis (D.V. Baxter) HHB-5320-Sp USA, Montana KC585252 KC585074 Vampola & Pouzar HHB-5298 USA, Montana − AY333829 HHB-12513 USA, Alaska AY966451 AY333830 -

High Diversity of Fungi Recovered from the Roots of Mature Tanoak (Lithocarpus Densiflorus) in Northern California
1380 High diversity of fungi recovered from the roots of mature tanoak (Lithocarpus densiflorus)in northern California S.E. Bergemann and M. Garbelotto Abstract: We collected mature tanoak (Lithocarpus densiflorus (Hook. & Arn.) Rehder) roots from five stands to charac- terize the relative abundance and taxonomic richness of root-associated fungi. Fungi were identified using polymerase chain reaction (PCR), cloning, and sequencing of internal transcribed spacer (ITS) and 28S rDNA. A total of 382 cloned PCR inserts were successfully sequenced and then classified into 119 taxa. Of these taxa, 82 were basidiomycetes, 33 were ascomycetes, and 4 were zygomycetes. Thirty-one of the ascomycete sequences were identified as Cenococcum geo- philum Fr. with overall richness of 22 ITS types. Other ascomycetes that form mycorrhizal associations were identified in- cluding Wilcoxina and Tuber as well as endophytes such as Lachnum, Cadophora, Phialophora, and Phialocephela. The most abundant mycorrhizal groups were Russulaceae (Lactarius, Macowanites, Russula) and species in the Thelephorales (Bankera, Boletopsis, Hydnellum, Tomentella). Our study demonstrates that tanoak supports a high diversity of ectomycor- rhizal fungi with comparable species richness to that observed in Quercus root communities. Key words: Cenoccocum geophilum, community, dark septate endophytes, ectomycorrhiza, species richness. Re´sume´ : Les auteurs ont pre´leve´ des racines de Lithocarpus densiflorus (Hook. & Arn.) Rehder) dans cinq peuplements, afin de caracte´riser l’abondance relative et la richesse taxonomique des champignons associe´sa` ses racines. On a identifie´ les champignons a` l’aide du PCR, par clonage et se´quenc¸age de l’ITS et du 28S rADN. On a se´quence´ avec succe`s 382 segments clone´s par PCR avant de les classifier en 119 taxons. -

Annotated Check List and Host Index Arizona Wood
Annotated Check List and Host Index for Arizona Wood-Rotting Fungi Item Type text; Book Authors Gilbertson, R. L.; Martin, K. J.; Lindsey, J. P. Publisher College of Agriculture, University of Arizona (Tucson, AZ) Rights Copyright © Arizona Board of Regents. The University of Arizona. Download date 28/09/2021 02:18:59 Link to Item http://hdl.handle.net/10150/602154 Annotated Check List and Host Index for Arizona Wood - Rotting Fungi Technical Bulletin 209 Agricultural Experiment Station The University of Arizona Tucson AÏfJ\fOTA TED CHECK LI5T aid HOST INDEX ford ARIZONA WOOD- ROTTlNg FUNGI /. L. GILßERTSON K.T IyIARTiN Z J. P, LINDSEY3 PRDFE550I of PLANT PATHOLOgY 2GRADUATE ASSISTANT in I?ESEARCI-4 36FZADAATE A5 S /STANT'" TEACHING Z z l'9 FR5 1974- INTRODUCTION flora similar to that of the Gulf Coast and the southeastern United States is found. Here the major tree species include hardwoods such as Arizona is characterized by a wide variety of Arizona sycamore, Arizona black walnut, oaks, ecological zones from Sonoran Desert to alpine velvet ash, Fremont cottonwood, willows, and tundra. This environmental diversity has resulted mesquite. Some conifers, including Chihuahua pine, in a rich flora of woody plants in the state. De- Apache pine, pinyons, junipers, and Arizona cypress tailed accounts of the vegetation of Arizona have also occur in association with these hardwoods. appeared in a number of publications, including Arizona fungi typical of the southeastern flora those of Benson and Darrow (1954), Nichol (1952), include Fomitopsis ulmaria, Donkia pulcherrima, Kearney and Peebles (1969), Shreve and Wiggins Tyromyces palustris, Lopharia crassa, Inonotus (1964), Lowe (1972), and Hastings et al. -

Appendix K. Survey and Manage Species Persistence Evaluation
Appendix K. Survey and Manage Species Persistence Evaluation Establishment of the 95-foot wide construction corridor and TEWAs would likely remove individuals of H. caeruleus and modify microclimate conditions around individuals that are not removed. The removal of forests and host trees and disturbance to soil could negatively affect H. caeruleus in adjacent areas by removing its habitat, disturbing the roots of host trees, and affecting its mycorrhizal association with the trees, potentially affecting site persistence. Restored portions of the corridor and TEWAs would be dominated by early seral vegetation for approximately 30 years, which would result in long-term changes to habitat conditions. A 30-foot wide portion of the corridor would be maintained in low-growing vegetation for pipeline maintenance and would not provide habitat for the species during the life of the project. Hygrophorus caeruleus is not likely to persist at one of the sites in the project area because of the extent of impacts and the proximity of the recorded observation to the corridor. Hygrophorus caeruleus is likely to persist at the remaining three sites in the project area (MP 168.8 and MP 172.4 (north), and MP 172.5-172.7) because the majority of observations within the sites are more than 90 feet from the corridor, where direct effects are not anticipated and indirect effects are unlikely. The site at MP 168.8 is in a forested area on an east-facing slope, and a paved road occurs through the southeast part of the site. Four out of five observations are more than 90 feet southwest of the corridor and are not likely to be directly or indirectly affected by the PCGP Project based on the distance from the corridor, extent of forests surrounding the observations, and proximity to an existing open corridor (the road), indicating the species is likely resilient to edge- related effects at the site. -

Blood Mushroom
Bleeding-Tooth Fungus Hydnellum Peckii Genus: Hydnellum Family: Bankeraceae Also known as: Strawberries and Cream Fungus, Bleeding Hydnellum, Red-Juice Tooth, or Devil’s Tooth. If you occasionally enjoy an unusual or weird sight in nature, we have one for you. Bleeding-Tooth Fungus fits this description with its strange colors and textures. This fungus is not toxic, but it is considered inedible because of its extremely bitter taste. Hydnoid species of fungus produce their spores on spines or “teeth”; these are reproductive structures. This fungus “bleeds” bright red droplets down the spines, so that it looks a little like blood against the whitish fungus. This liquid actually has an anticoagulant property similar to the medicine heparin; it keeps human or animal blood from clotting. This fungus turns brown with age. Bloody-Tooth Fungus establishes a relationship with the roots of certain trees, so you will find it lower down on the tree’s trunk. The fungus exchanges the minerals and amino acids it has extracted from the soil with its enzymes, for oxygen and carbon within the host tree that allow the fungus to flourish. It’s a great partnership that benefits both, called symbiosis. The picture above was taken at Kings Corner at the pine trees on the west side of the property. It was taken in early to mid-autumn. This part of the woods is moist enough to grow some really beautiful mushrooms and fungi. Come and see—but don’t touch or destroy. Fungi should be respected for the role they play in the woods ecology. -

Colonisation of Pinus Radiata Thinning Stumps by Armillaria and Other Basidiomycetes Following Treatment with Armillaria Basidiospores
COLONISATION OF PINUS RADIATA THINNING STUMPS BY ARMILLARIA AND OTHER BASIDIOMYCETES FOLLOWING TREATMENT WITH ARMILLARIA BASIDIOSPORES *I.A. Hood and *J.F. Gardner *New Zealand Forest Research Institute, Rotorua, New Zealand Abstract Stumps of thinned trees and partly buried stem segments were treated with basidiospores of two Armillaria species, to test the possibility that colonisation of woody material by basidiospores serves to spread Armillaria species into Pinus radiata plantations in New Zealand. Spore colonisation was confirmed for segments and demonstrated for the first time on freshly cut pine stumps. Effective invasion occurred at high spore concentrations and was apparent from 6 months after felling. Other basidiomycetes were first isolated from stumps at 6 months and were consistently cultured between 1 and 3 years following felling, after which yields became irregular due to stump deterioration and increasing contamination. Certain basidiomycetes were isolated regularly whereas many others were cultured only infrequently. A culture bank was established of basidiomycetes to be tested as candidates for potential biological control of A. novae-zelandiae in pine stumps. Introduction It is important to know whether Armillaria novae-zelandiae (Stevenson) Herink is spreading into stands of Pinus radiata D. Don by means of basidiospores dispersed from fruitbodies in nearby indigenous forests in New Zealand. The formation of new, spore-derived colonies is inferred from the high densities of A. novae- zelandiae genets in second rotation plantations on sites not originally stocked in native forest. However, direct supporting evidence consists so far only of the demonstrated colonisation of freshly cut partly buried pine billets employed as spore traps (Hood et al ., 2002). -

Cultural Characterization and Chlamydospore Function of the Ganodermataceae Present in the Eastern United States
Mycologia ISSN: 0027-5514 (Print) 1557-2536 (Online) Journal homepage: https://www.tandfonline.com/loi/umyc20 Cultural characterization and chlamydospore function of the Ganodermataceae present in the eastern United States Andrew L. Loyd, Eric R. Linder, Matthew E. Smith, Robert A. Blanchette & Jason A. Smith To cite this article: Andrew L. Loyd, Eric R. Linder, Matthew E. Smith, Robert A. Blanchette & Jason A. Smith (2019): Cultural characterization and chlamydospore function of the Ganodermataceae present in the eastern United States, Mycologia To link to this article: https://doi.org/10.1080/00275514.2018.1543509 View supplementary material Published online: 24 Jan 2019. Submit your article to this journal View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=umyc20 MYCOLOGIA https://doi.org/10.1080/00275514.2018.1543509 Cultural characterization and chlamydospore function of the Ganodermataceae present in the eastern United States Andrew L. Loyd a, Eric R. Lindera, Matthew E. Smith b, Robert A. Blanchettec, and Jason A. Smitha aSchool of Forest Resources and Conservation, University of Florida, Gainesville, Florida 32611; bDepartment of Plant Pathology, University of Florida, Gainesville, Florida 32611; cDepartment of Plant Pathology, University of Minnesota, St. Paul, Minnesota 55108 ABSTRACT ARTICLE HISTORY The cultural characteristics of fungi can provide useful information for studying the biology and Received 7 Feburary 2018 ecology of a group of closely related species, but these features are often overlooked in the order Accepted 30 October 2018 Polyporales. Optimal temperature and growth rate data can also be of utility for strain selection of KEYWORDS cultivated fungi such as reishi (i.e., laccate Ganoderma species) and potential novel management Chlamydospores; tactics (e.g., solarization) for butt rot diseases caused by Ganoderma species. -

Phylogenetic Classification of Trametes
TAXON 60 (6) • December 2011: 1567–1583 Justo & Hibbett • Phylogenetic classification of Trametes SYSTEMATICS AND PHYLOGENY Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset Alfredo Justo & David S. Hibbett Clark University, Biology Department, 950 Main St., Worcester, Massachusetts 01610, U.S.A. Author for correspondence: Alfredo Justo, [email protected] Abstract: The phylogeny of Trametes and related genera was studied using molecular data from ribosomal markers (nLSU, ITS) and protein-coding genes (RPB1, RPB2, TEF1-alpha) and consequences for the taxonomy and nomenclature of this group were considered. Separate datasets with rDNA data only, single datasets for each of the protein-coding genes, and a combined five-marker dataset were analyzed. Molecular analyses recover a strongly supported trametoid clade that includes most of Trametes species (including the type T. suaveolens, the T. versicolor group, and mainly tropical species such as T. maxima and T. cubensis) together with species of Lenzites and Pycnoporus and Coriolopsis polyzona. Our data confirm the positions of Trametes cervina (= Trametopsis cervina) in the phlebioid clade and of Trametes trogii (= Coriolopsis trogii) outside the trametoid clade, closely related to Coriolopsis gallica. The genus Coriolopsis, as currently defined, is polyphyletic, with the type species as part of the trametoid clade and at least two additional lineages occurring in the core polyporoid clade. In view of these results the use of a single generic name (Trametes) for the trametoid clade is considered to be the best taxonomic and nomenclatural option as the morphological concept of Trametes would remain almost unchanged, few new nomenclatural combinations would be necessary, and the classification of additional species (i.e., not yet described and/or sampled for mo- lecular data) in Trametes based on morphological characters alone will still be possible. -

Hydne Tomenteux
Hydne tomenteux Comestible, sans valeur Recommandation officielle: Nom latin: Phellodon tomentosus Famille: A aiguillons > Bankeraceae > Phellodon Caractéristiques du genre Phellodon : chapeau: face intérieure du chapeau couverte d'aiguillons élastiques, chair liégeuse, zonée à l'intérieur, odeur (sec) de bouillon de viande ou livèche - lames: aiguillons élastiques - pied: existant mais court - remarques: saprophyte Synonymes: Calodon tomentosus, Calodon cyathiformis, Phellodon cyathiformis, Hydnum leptopus, Hydnum tomentosum Chapeau: 2-6cm, étalé, déprimé, largement infundibuliforme à ombiliqué, sec, lisse à ridulé radialement, squamuleux-hérissé au centre, tomenteux à fibrilleux, feutré, vers la marge, blanc au tout début, typiquement fortement zoné de jaune-brun, brun pâle à foncé, gris-brun à brun rougeâtre, demeurant souvent blanc vers le pourtour, à marge se tachant de brunâtre au froissement Lamelles: sans lames, aiguillons courts, subdécurrents, serrés, blancs puis chamois, grisâtres à gris-brun cendré, plus pâles aux bouts, brunissant ou tachant de chamois vineux au froissement Pied: souvent centré, égal à atténué vers la base, lisse à subfibrilleux, concolore au chapeau, orné de bandelettes crème, brunes à bleutées, arrivant d'une couche mycélienne feutrée brunâtre Chair: coriace-fibreux, brunâtre dans le chapeau, zoné et avec couche externe subéreuse tan brunâtre et couche interne brun foncé dans le pied Odeur: aromatisée, sucrée ou de fenugrec, foin frais, surtout au sec sur le chapeau, spermatique sur les aiguillons Saveur: douce à amarescente, parfois un peu piquante Habitat: août-octobre, dispersé, cespiteux ou souvent en troupes qui se fondent les uns dans les autres, mycorhizien, sur sol des forêts de conifères et pins, appréciant la bruyère et les terres acides, assez rare Remarques: Ses pieds se séparent même si plusieurs chapeaux sont fusionnés ensemble Confusion: peu de risque de confusion avec des champignons que l'on trouve en Suisse romande. -

Basidiomycota) in Finland
Mycosphere 7 (3): 333–357(2016) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/7/3/7 Copyright © Guizhou Academy of Agricultural Sciences Extensions of known geographic distribution of aphyllophoroid fungi (Basidiomycota) in Finland Kunttu P1, Kulju M2, Kekki T3, Pennanen J4, Savola K5, Helo T6 and Kotiranta H7 1University of Eastern Finland, School of Forest Sciences, P.O. Box 111, FI-80101 Joensuu, Finland 2Biodiversity Unit P.O. Box 3000, FI-90014 University of Oulu, Finland 3Jyväskylä University Museum, Natural History Section, P.O. BOX 35, FI-40014 University of Jyväskylä, Finland 4Pentbyntie 1 A 2, FI-10300 Karjaa, Finland 5The Finnish Association for Nature Conservation, Itälahdenkatu 22 b A, FI-00210 Helsinki, Finland 6Erätie 13 C 19, FI-87200 Kajaani, Finland 7Finnish Environment Institute, P.O. Box 140, FI-00251 Helsinki, Finland Kunttu P, Kulju M, Kekki T, Pennanen J, Savola K, Helo T, Kotiranta H 2016 – Extensions of known geographic distribution of aphyllophoroid fungi (Basidiomycota) in Finland. Mycosphere 7(3), 333–357, Doi 10.5943/mycosphere/7/3/7 Abstract This article contributes the knowledge of Finnish aphyllophoroid funga with nationally or regionally new species, and records of rare species. Ceriporia bresadolae, Clavaria tenuipes and Renatobasidium notabile are presented as new aphyllophoroid species to Finland. Ceriporia bresadolae and R. notabile are globally rare species. The records of Ceriporia aurantiocarnescens, Crustomyces subabruptus, Sistotrema autumnale, Trechispora elongata, and Trechispora silvae- ryae are the second in Finland. New records (or localities) are provided for 33 species with no more than 10 records in Finland. In addition, 76 records of aphyllophoroid species are reported as new to some subzones of the boreal vegetation zone in Finland. -

A Phylogenetic Overview of the Antrodia Clade (Basidiomycota, Polyporales)
Mycologia, 105(6), 2013, pp. 1391–1411. DOI: 10.3852/13-051 # 2013 by The Mycological Society of America, Lawrence, KS 66044-8897 A phylogenetic overview of the antrodia clade (Basidiomycota, Polyporales) Beatriz Ortiz-Santana1 phylogenetic studies also have recognized the genera Daniel L. Lindner Amylocystis, Dacryobolus, Melanoporia, Pycnoporellus, US Forest Service, Northern Research Station, Center for Sarcoporia and Wolfiporia as part of the antrodia clade Forest Mycology Research, One Gifford Pinchot Drive, (SY Kim and Jung 2000, 2001; Binder and Hibbett Madison, Wisconsin 53726 2002; Hibbett and Binder 2002; SY Kim et al. 2003; Otto Miettinen Binder et al. 2005), while the genera Antrodia, Botanical Museum, University of Helsinki, PO Box 7, Daedalea, Fomitopsis, Laetiporus and Sparassis have 00014, Helsinki, Finland received attention in regard to species delimitation (SY Kim et al. 2001, 2003; KM Kim et al. 2005, 2007; Alfredo Justo Desjardin et al. 2004; Wang et al. 2004; Wu et al. 2004; David S. Hibbett Dai et al. 2006; Blanco-Dios et al. 2006; Chiu 2007; Clark University, Biology Department, 950 Main Street, Worcester, Massachusetts 01610 Lindner and Banik 2008; Yu et al. 2010; Banik et al. 2010, 2012; Garcia-Sandoval et al. 2011; Lindner et al. 2011; Rajchenberg et al. 2011; Zhou and Wei 2012; Abstract: Phylogenetic relationships among mem- Bernicchia et al. 2012; Spirin et al. 2012, 2013). These bers of the antrodia clade were investigated with studies also established that some of the genera are molecular data from two nuclear ribosomal DNA not monophyletic and several modifications have regions, LSU and ITS. A total of 123 species been proposed: the segregation of Antrodia s.l.