Opsin Genes, Cone Photopigments, Color Vision, and Color Blindness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Introduction to the Chemistry of Dyes
GENERAL INTRODUCTION TO THE CHEMISTRY OF DYES 1. Principles of Colour Chemistry 1.1 Basis for colour Unlike most organic compounds, dyes possess colour because they 1) absorb light in the visible spectrum (400–700 nm), 2) have at least one chromophore (colour-bearing group), 3) have a conjugated system, i.e. a structure with alternating double and single bonds, and 4) exhibit resonance of electrons, which is a stabilizing force in organic compounds (Abrahart, 1977). When any one of these features is lacking from the molecular structure the colour is lost. In addition to chromophores, most dyes also contain groups known as auxochromes (colour helpers), examples of which are carboxylic acid, sulfonic acid, amino, and hydroxyl groups. While these are not responsible for colour, their presence can shift the colour of a colourant and they are most often used to influence dye solubility. Figure 1 shows the relationships between wavelength of visible and colour absorbed/observed. Other factors contributing to colour are illustrated in Figures 2–4. Fig. 1. Wavelength of light absorption versus colour in organic dyes Wavelength Absorbed (nm) Colour Absorbed Colour Observed 400–435 Violet Yellow-Green 435–480 Blue Yellow 480–490 Green-Blue Orange 490–500 Blue-Green Red 500–560 Green Purple 560–580 Yellow-Green Violet 580–595 Yellow Blue 595–605 Orange Green-Blue 605–700 Red Blue-Green –55– 56 IARC MONOGRAPHS VOLUME 99 Fig. 2. Examples of chromophoric groups present in organic dyes O O N N N N O N N O H N Anthraquinone H N Nitro Azo N N Ar C N N C Ar Methine Phthalocyanine Triarylmethane Fig. -

Blue Cone Monochromacy: Visual Function and Efficacy Outcome Measures for Clinical Trials
RESEARCH ARTICLE Blue Cone Monochromacy: Visual Function and Efficacy Outcome Measures for Clinical Trials Xunda Luo1☯‡, Artur V. Cideciyan1☯‡*, Alessandro Iannaccone2, Alejandro J. Roman1, Lauren C. Ditta2, Barbara J. Jennings2, Svetlana A. Yatsenko3, Rebecca Sheplock1, Alexander Sumaroka1, Malgorzata Swider1, Sharon B. Schwartz1, Bernd Wissinger4, Susanne Kohl4, Samuel G. Jacobson1* 1 Scheie Eye Institute, Department of Ophthalmology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America, 2 Hamilton Eye Institute, Department of Ophthalmology, University of Tennessee Health Science Center, Memphis, Tennessee, United States of America, 3 Pittsburgh Cytogenetics Laboratory, Center for Medical Genetics and Genomics, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, United States of America, 4 Molecular Genetics Laboratory, Institute for Ophthalmic Research, Centre for Ophthalmology, University of Tuebingen, Tuebingen, Germany ☯ These authors contributed equally to this work. ‡ OPEN ACCESS These authors are joint first authors on this work. * [email protected] (SGJ); [email protected] (AVC) Citation: Luo X, Cideciyan AV, Iannaccone A, Roman AJ, Ditta LC, Jennings BJ, et al. (2015) Blue Cone Monochromacy: Visual Function and Efficacy Abstract Outcome Measures for Clinical Trials. PLoS ONE 10(4): e0125700. doi:10.1371/journal.pone.0125700 Academic Editor: Dror Sharon, Hadassah-Hebrew University Medical Center, ISRAEL Background Blue Cone Monochromacy (BCM) is an X-linked retinopathy caused by mutations in the Received: December 29, 2014 OPN1LW / OPN1MW gene cluster, encoding long (L)- and middle (M)-wavelength sensitive Accepted: March 21, 2015 cone opsins. Recent evidence shows sufficient structural integrity of cone photoreceptors in Published: April 24, 2015 BCM to warrant consideration of a gene therapy approach to the disease. -

Color Blindness LEVELED BOOK • W a Reading A–Z Level W Leveled Book Word Count: 1,349 Color Blindness Connections Writing Choose Two Forms of Color Blindness
Color Blindness LEVELED BOOK • W A Reading A–Z Level W Leveled Book Word Count: 1,349 Color Blindness Connections Writing Choose two forms of color blindness. Write a report that compares the two conditions and their effects on a person’s life. Math Research the statistics about the number of people with the various forms of color blindness in your country. Organize your results in a pie chart. • W Q •T Written by Cheryl Reifsnyder Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com Words to Know Color ancestry molecules complementary photopigments cone cells prism Blindness defects retina genetic disorder rod cells hereditary wavelengths Photo Credits: Front cover, Back cover: © pretoperola/123RF; title page (Both): © shironosov/ iStock/Thinkstock; page 3: © Véronique Burger/Science Source; page 4 (Both): © Michael Shake/Dreamstime.com; page 5: © Designua/Dreamstime.com; page 6 (top): © Gunilla Elam/Science Source; page 6 (Bottom): © Steve Gschmeissner/Science Source; page 7 (Both): © Ted Kinsman/ Science Source; page 8 (left, Both): © anatchant/iStock/Thinkstock; page 8 (center, Both): © Nadezhda Bolotina/Hemera/Thinkstock; page 8 (right, Both): © iremphotography/iStock/Thinkstock; page 9 (all): © Popartic/iStock Editorial/Thinkstock; page 10: © RVN/Alamy Stock Photo; page 12: © Alexander Kaludov/123RF; page 13: © EnChroma; page 14: © Neitz LaBoratory; page 15: © Phanie/Alamy Stock Photo Written by Cheryl Reifsnyder www.readinga-z.com Focus Question Color Blindness Level W Leveled Book Correlation © Learning A–Z LEVEL W Written By Cheryl Reifsnyder What causes color blindness, and how Fountas & Pinnell S can it affect a person’s life? All rights reserved. -

TETRACHROMATIC COLOR VISION Kimberly A. Jameson Prepared For
TETRACHROMATIC COLOR VISION Kimberly A. Jameson prepared for The Oxford Companion to Consciousness. Wilken, P., Bayne, T. & Cleeremans, A. (Ed.s). Oxford University Press: Oxford. To appear in 2007. The term“tetrachromacy” describes the physiological possession of four different classes of simultaneously functioning retinal photopigments (also called “weak tetrachromacy”). From an empirical standpoint, tetrachromatic color vision (or “strong tetrachromacy”) additionally requires demonstrating that mixtures of four independent appropriately chosen primary lights will simulate all distinc- tions in appearance possible in visible color space. Independence of the primary lights implies that no mixtures of any subset of these lights (or their intensity variants) will produce an identical match to any combination of mixtures of the remaining lights. By comparison, trichromacy empirically requires only three primaries to simulate all visible colors. Established theory states that normal color vision humans are trichromats (as, primarily, are Old World monkeys and apes). The first element of trichro- macy is the output from three simultaneously functioning retinal cone classes: Short-, Medium-, and Long- Wavelenth Sensitive (SWS, MWS, & LWS) cones. Three cone classes alone do not establish a trichromat color code, however. A postreceptoral code for three categories of signal is also needed. A standard vision science assumption is that the postreceptoral recoding of cone outputs initiates the neural trivariant (or trichromatic) property of human color percep- tion, and the need for only three primary lights to match any test light. ANIMAL TETRACHROMACY: Tetrachromacy is an early vertebrate characteristic, existing in fish and reptiles, and is evolutionarily more ancient than primate trichromacy. Essentially all diu- ral birds have four retinal cone types (two SWS classes, plus a MWS and a LWS class) which neurally produce four dimensional color experience, or tetrachro- matic color vision. -
RETINAL DISORDERS Eye63 (1)
RETINAL DISORDERS Eye63 (1) Retinal Disorders Last updated: May 9, 2019 CENTRAL RETINAL ARTERY OCCLUSION (CRAO) ............................................................................... 1 Pathophysiology & Ophthalmoscopy ............................................................................................... 1 Etiology ............................................................................................................................................ 2 Clinical Features ............................................................................................................................... 2 Diagnosis .......................................................................................................................................... 2 Treatment ......................................................................................................................................... 2 BRANCH RETINAL ARTERY OCCLUSION ................................................................................................ 3 CENTRAL RETINAL VEIN OCCLUSION (CRVO) ..................................................................................... 3 Pathophysiology & Etiology ............................................................................................................ 3 Clinical Features ............................................................................................................................... 3 Diagnosis ......................................................................................................................................... -

Identification and Distribution of Dietary Precursors of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 39 (1999) 219–229 Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC David R. Giovannucci *, Robert S. Stephenson Department of Biological Sciences, Wayne State Uni6ersity, Detroit, MI 48202, USA Received 3 July 1997; received in revised form 10 November 1997 Abstract A dietary source of retinoid or carotenoid has been shown to be necessary for the biosynthesis of functional visual pigment in flies. In the present study, the larvae or adults of Drosophila melanogaster were administered specific carotenoid-containing diets and high performance liquid chromatography was used to identify and quantify the carotenoids in extracts of wild type and ninaD visual mutant flies. When b-carotene was fed to larvae, wild type flies were shown to hydroxylate this molecule and to accumulate zeaxanthin and a small amount of b-cryptoxanthin. Zeaxanthin content was found to increase throughout development and was a major carotenoid peak detected in the adult fly. Carotenoids were twice as effective at mediating zeaxanthin accumulation when provided to larvae versus adults. In the ninaD mutant, zeaxanthin content was shown to be specifically and significantly altered compared to wild type, and was ineffective at mediating visual pigment synthesis when provided to both larval and adult mutant flies. It is proposed that zeaxanthin is the larval storage form for subsequent visual pigment chromophore biosynthesis during pupation, that zeaxanthin or b-crytoxanthin is the immediate precursor for light-independent chromophore synthesis in the adult, and that the ninaD mutant is defective in this pathway. -
Chapter 6 COLOR and COLOR VISION
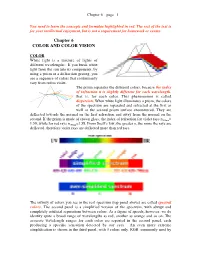
Chapter 6 – page 1 You need to learn the concepts and formulae highlighted in red. The rest of the text is for your intellectual enjoyment, but is not a requirement for homework or exams. Chapter 6 COLOR AND COLOR VISION COLOR White light is a mixture of lights of different wavelengths. If you break white light from the sun into its components, by using a prism or a diffraction grating, you see a sequence of colors that continuously vary from red to violet. The prism separates the different colors, because the index of refraction n is slightly different for each wavelength, that is, for each color. This phenomenon is called dispersion. When white light illuminates a prism, the colors of the spectrum are separated and refracted at the first as well as the second prism surface encountered. They are deflected towards the normal on the first refraction and away from the normal on the second. If the prism is made of crown glass, the index of refraction for violet rays n400nm= 1.59, while for red rays n700nm=1.58. From Snell’s law, the greater n, the more the rays are deflected, therefore violet rays are deflected more than red rays. The infinity of colors you see in the real spectrum (top panel above) are called spectral colors. The second panel is a simplified version of the spectrum, with abrupt and completely artificial separations between colors. As a figure of speech, however, we do identify quite a broad range of wavelengths as red, another as orange and so on. -

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease. -

OCULAR GENE THERAPY TRIALS ADVANCE Results of the First Phase 3 Trial Were Announced
OCULAR GENE THERAPY TRIALS ADVANCE Results of the first phase 3 trial were announced. BY ARON SHAPIRO INNOVATIONS IN RETINA INNOVATIONS “We used to think that our fate was in our AAV FACILITATES GENE DELIVERY stars, but now we know that, in large measure, The nonpathogenic adeno-associated virus (AAV) has our fate is in our genes.” to date been a safe and effective vector for gene delivery. –James Watson1 The recombinant AAV (rAAV) vector has demonstrated increased specificity and efficiency in ocular AAV-mediated Genetic alterations are known to be respon- gene therapy interventions. Recombinant AAV2 (rAAV2) sible for numerous diseases, and so it follows vectors used for gene therapy are derived from the wild-type logically that the best cures for these diseases virus by deleting the entire viral coding region and replac- might lie in correcting genetic anomalies by the process of ing it with the reporter or therapeutic transgene. Combined gene therapy. This approach involves the introduction of AAV serotypes have also been developed. This diversity genes into existing cells in attempts to prevent or cure a of serotypes may lead to more specific and more efficient wide range of diseases previously thought to be incurable.1 transduction. However, successful and efficient transfection Posterior segment disorders are challenging to treat, and of particular cell types in the eye still depends on other fac- current therapies have numerous shortcomings. Many are tors, such as the AAV titer, the site of injection, the amount invasive, run the risk of complications, offer only short-term of passenger DNA, and the specific gene promoters where relief from symptoms, or are unable to directly treat vision transcription initiation takes place.2 loss. -

Updates on Myopia
Updates on Myopia A Clinical Perspective Marcus Ang Tien Y. Wong Editors Updates on Myopia Marcus Ang • Tien Y. Wong Editors Updates on Myopia A Clinical Perspective Editors Marcus Ang Tien Y. Wong Singapore National Eye Center Singapore National Eye Center Duke-NUS Medical School Duke-NUS Medical School National University of Singapore National University of Singapore Singapore Singapore This book is an open access publication. ISBN 978-981-13-8490-5 ISBN 978-981-13-8491-2 (eBook) https://doi.org/10.1007/978-981-13-8491-2 © The Editor(s) (if applicable) and The Author(s) 2020, corrected publication 2020 Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specifc statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Color Vision Test for Dichromatic and Trichromatic Macaque Monkeys
Journal of Vision (2013) 13(13):1, 1–15 http://www.journalofvision.org/content/13/13/1 1 Color vision test for dichromatic and trichromatic macaque monkeys National Institute for Physiological Sciences, Okazaki, Aichi, Japan Electronics-Inspired Interdisciplinary Research Institute, Toyohashi University of Technology, Toyohashi, Aichi, # Kowa Koida* Japan $ National Institute for Physiological Sciences, Okazaki, Aichi, Japan University for Advanced Studies (SOKENDAI), Okazaki, # Isao Yokoi* Aichi, Japan $ National Institute for Physiological Sciences, Okazaki, Aichi, Japan University for Advanced Studies (SOKENDAI), Okazaki, # Gouki Okazawa Aichi, Japan $ Faculty of Rehabilitation, Chubu Gakuin University, Seki, # Akichika Mikami Gifu, Japan $ Department of Biology, Bogor Agricultural University, # Kanthi Arum Widayati Bogor, Indonesia $ Primate Research Institute, Kyoto University, Inuyama, # Shigehiro Miyachi Aichi, Japan $ National Institute for Physiological Sciences, Okazaki, Aichi, Japan University for Advanced Studies (SOKENDAI), Okazaki, # Hidehiko Komatsu Aichi, Japan $ Dichromacy is a color vision defect in which one of the identified dichromatic macaques (Macaca fascicularis) three cone photoreceptors is absent. Individuals with with that of normal trichromatic macaques. In the color dichromacy are called dichromats (or sometimes ‘‘color- discrimination test, dichromats could not discriminate blind’’), and their color discrimination performance has colors along the protanopic confusion line, though contributed significantly -

Human Trichromacy Revisited PNAS PLUS
Human trichromacy revisited PNAS PLUS Hiroshi Horiguchia,b,1, Jonathan Winawera, Robert F. Doughertyc, and Brian A. Wandella,c aPsychology Department, Stanford University, Stanford, CA 94305; bDepartment of Ophthalmology, School of Medicine, Jikei University, Tokyo 105-8461, Japan; and cStanford Center for Cognitive and Neurobiological Imaging, Stanford, CA 94305 AUTHOR SUMMARY The history of trichromatic color As predicted by the classic tri- theory spans nearly 300 y, be- msARC Fovea Periphery chromatic model, subjects fail to ’ Opticks (%) ginning with Newton s 10 see cone-silent stimuli presented (1). Thomas Young’s Royal So- Visible in the fovea. In contrast, subjects Not ciety Lecture (2) explained how 0 Not do see cone-silent stimuli pre- Visible Visible Visible Visible color perception is initiated by S1 sented to the peripheral visual S2 fi the absorption of light in three Cone-silent −10 eld. In extensive computational S3 fi types of human light receptors, −10 0 10 −10 0 10 (%) modeling, we could nd no plau- the cones. The theory of tri- S-cone S-cone sible pigment density adjustment chromacy is the foundation of (including the lens or photopig- modern color technologies (3). Fig. P1. Built and found. (A) An apparatus using six primary lights was ment densities) that explains the built for the experiment. Mean luminance through the eyepiece was measurements in the periphery For more than a century, the 2 absorption of light in the retina 2,060 cd/m .(B) Graphs represent detection thresholds for stimuli that based on a model that includes was thought to occur only in the have some S-cone contrast and cone-silent contrast, but no L- or M- only three cone photopigments.