Download Full Report 13.6MB .Pdf File
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

A Revision of the Palaeocorystoidea and the Phylogeny of Raninoidian Crabs (Crustacea, Decapoda, Brachyura, Podotremata)
Zootaxa 3215: 1–216 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) ZOOTAXA 3215 A revision of the Palaeocorystoidea and the phylogeny of raninoidian crabs (Crustacea, Decapoda, Brachyura, Podotremata) BARRY W.M. VAN BAKEL1, 6, DANIÈLE GUINOT2, PEDRO ARTAL3, RENÉ H.B. FRAAIJE4 & JOHN W.M. JAGT5 1 Oertijdmuseum De Groene Poort, Bosscheweg 80, NL–5283 WB Boxtel, the Netherlands; and Nederlands Centrum voor Biodiver- siteit [Naturalis], P.O. Box 9517, NL–2300 RA Leiden, the Netherlands E-mail: [email protected] 2 Département Milieux et peuplements aquatiques, Muséum national d'Histoire naturelle, 61 rue Buffon, CP 53, F–75231 Paris Cedex 5, France E-mail: [email protected] 3 Museo Geológico del Seminario de Barcelona, Diputación 231, E–08007 Barcelona, Spain E-mail: [email protected] 4 Oertijdmuseum De Groene Poort, Bosscheweg 80, NL–5283 WB Boxtel, the Netherlands E-mail: [email protected] 5 Natuurhistorisch Museum Maastricht, de Bosquetplein 6–7, NL–6211 KJ Maastricht, the Netherlands E-mail: [email protected] 6 Corresponding author Magnolia Press Auckland, New Zealand Accepted by P. Castro: 2 Dec. 2011; published: 29 Feb. 2012 BARRY W.M. VAN BAKEL, DANIÈLE GUINOT, PEDRO ARTAL, RENÉ H.B. FRAAIJE & JOHN W.M. JAGT A revision of the Palaeocorystoidea and the phylogeny of raninoidian crabs (Crustacea, Deca- poda, Brachyura, Podotremata) (Zootaxa 3215) 216 pp.; 30 cm. 29 Feb. 2012 ISBN 978-1-86977-873-6 (paperback) ISBN 978-1-86977-874-3 (Online edition) FIRST PUBLISHED IN 2012 BY Magnolia Press P.O. -

Resources on the South Coast of Western Australia
Evaluation of Crystal Crab (Chaceon bicolor) resources on the south coast of Western Australia Chuwen, B.M. and R. Stevens Project No. 2003/077 Fisheries Research and Development Corporation Report FRDC project 2003/077 Fisheries Research and Development Corporation Report FRDC project 2003/077 FINAL REPORT Evaluation of Crystal Crab (Chaceon bicolor) resources on the south coast of Western Australia Chuwen, B.M. Stevens, R. May 2006 Western Australian Fishing Industry Council Suite 6, 41 Walters Drive Osborne Park WA 6017 Copyright Fisheries Research and Development Corporation and Western Australian Fishing Industry Council 2006 This work is copyright. Except as permitted under the Copyright Act 1968 (Cth), no part of this publication may be reproduced by any process, electronic or otherwise, without the specific written permission of the copyright owners. Neither may information be stored electronically in any form whatsoever without such permission. The Fisheries Research and Development Corporation plans, invests in and manages fisheries research and development throughout Australia. It is a statutory authority within the portfolio of the federal Minister for Agriculture, Fisheries and Forestry, jointly funded by the Australian Government and the fishing industry. ISBN: 0-86905 876 2 1 Table of Contents NON TECHNICAL SUMMARY ..................................................................................................... 4 ACKNOWLEDGMENTS ................................................................................................................ -
W7192e19.Pdf
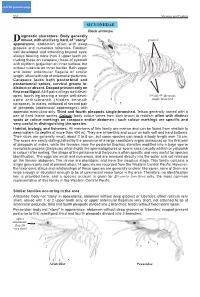
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Skates and Rays Diversity, Exploration and Conservation – Case-Study of the Thornback Ray, Raja Clavata
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Doutoramento em Ciências do Mar 2010 UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Tese orientada por Professor Auxiliar com Agregação Leonel Serrano Gordo e Investigadora Auxiliar Ivone Figueiredo Doutoramento em Ciências do Mar 2010 The research reported in this thesis was carried out at the Instituto de Investigação das Pescas e do Mar (IPIMAR - INRB), Unidade de Recursos Marinhos e Sustentabilidade. This research was funded by Fundação para a Ciência e a Tecnologia (FCT) through a PhD grant (SFRH/BD/23777/2005) and the research project EU Data Collection/DCR (PNAB). Skates and rays diversity, exploration and conservation | Table of Contents Table of Contents List of Figures ............................................................................................................................. i List of Tables ............................................................................................................................. v List of Abbreviations ............................................................................................................. viii Agradecimentos ........................................................................................................................ -

Decapod Crustacean Assemblages Off the West Coast of Central Italy (Western Mediterranean)
SCIENTIA MARINA 71(1) March 2007, 19-28, Barcelona (Spain) ISSN: 0214-8358 Decapod crustacean assemblages off the West coast of central Italy (western Mediterranean) EMANUELA FANELLI 1, FRANCESCO COLLOCA 2 and GIANDOMENICO ARDIZZONE 2 1 IAMC-CNR Marine Ecology Laboratory, Via G. da Verrazzano 17, 91014 Castellammare del Golfo (Trapani) Italy. E-mail: [email protected] 2 Department of Animal and Human Biology, University of Rome “la Sapienza”, V.le dell’Università 32, 00185 Rome, Italy. SUMMARY: Community structure and faunal composition of decapod crustaceans off the west coast of central Italy (west- ern Mediterranean) were investigated. Samples were collected during five trawl surveys carried out from June 1996 to June 2000 from 16 to 750 m depth. Multivariate analysis revealed the occurrence of five faunistic assemblages: 1) a strictly coastal community over sandy bottoms at depths <35 m; 2) a middle shelf community over sandy-muddy bottoms at depths between 50 and 100 m; 3) a slope edge community up to 200 m depth as a transition assemblage; 4) an upper slope community at depths between 200 and 450 m, and 5) a middle slope community at depths greater than 450 m. The existence of a shelf- slope edge transition is a characteristic of the western and central Mediterranean where a Leptometra phalangium facies is found in many areas at depths between 120 and 180 m. The brachyuran crab Liocarcinus depurator dominates the shallow muddy-sandy bottoms of the shelf, while Parapenaeus longirostris is the most abundant species from the shelf to the upper slope assemblage. -

Checklists of Crustacea Decapoda from the Canary and Cape Verde Islands, with an Assessment of Macaronesian and Cape Verde Biogeographic Marine Ecoregions
Zootaxa 4413 (3): 401–448 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4413.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:2DF9255A-7C42-42DA-9F48-2BAA6DCEED7E Checklists of Crustacea Decapoda from the Canary and Cape Verde Islands, with an assessment of Macaronesian and Cape Verde biogeographic marine ecoregions JOSÉ A. GONZÁLEZ University of Las Palmas de Gran Canaria, i-UNAT, Campus de Tafira, 35017 Las Palmas de Gran Canaria, Spain. E-mail: [email protected]. ORCID iD: 0000-0001-8584-6731. Abstract The complete list of Canarian marine decapods (last update by González & Quiles 2003, popular book) currently com- prises 374 species/subspecies, grouped in 198 genera and 82 families; whereas the Cape Verdean marine decapods (now fully listed for the first time) are represented by 343 species/subspecies with 201 genera and 80 families. Due to changing environmental conditions, in the last decades many subtropical/tropical taxa have reached the coasts of the Canary Islands. Comparing the carcinofaunal composition and their biogeographic components between the Canary and Cape Verde ar- chipelagos would aid in: validating the appropriateness in separating both archipelagos into different ecoregions (Spalding et al. 2007), and understanding faunal movements between areas of benthic habitat. The consistency of both ecoregions is here compared and validated by assembling their decapod crustacean checklists, analysing their taxa composition, gath- ering their bathymetric data, and comparing their biogeographic patterns. Four main evidences (i.e. different taxa; diver- gent taxa composition; different composition of biogeographic patterns; different endemicity rates) support that separation, especially in coastal benthic decapods; and these parametres combined would be used as a valuable tool at comparing biotas from oceanic archipelagos. -

From Ghost and Mud Shrimp
Zootaxa 4365 (3): 251–301 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4365.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C5AC71E8-2F60-448E-B50D-22B61AC11E6A Parasites (Isopoda: Epicaridea and Nematoda) from ghost and mud shrimp (Decapoda: Axiidea and Gebiidea) with descriptions of a new genus and a new species of bopyrid isopod and clarification of Pseudione Kossmann, 1881 CHRISTOPHER B. BOYKO1,4, JASON D. WILLIAMS2 & JEFFREY D. SHIELDS3 1Division of Invertebrate Zoology, American Museum of Natural History, Central Park West @ 79th St., New York, New York 10024, U.S.A. E-mail: [email protected] 2Department of Biology, Hofstra University, Hempstead, New York 11549, U.S.A. E-mail: [email protected] 3Department of Aquatic Health Sciences, Virginia Institute of Marine Science, College of William & Mary, P.O. Box 1346, Gloucester Point, Virginia 23062, U.S.A. E-mail: [email protected] 4Corresponding author Table of contents Abstract . 252 Introduction . 252 Methods and materials . 253 Taxonomy . 253 Isopoda Latreille, 1817 . 253 Bopyroidea Rafinesque, 1815 . 253 Ionidae H. Milne Edwards, 1840. 253 Ione Latreille, 1818 . 253 Ione cornuta Bate, 1864 . 254 Ione thompsoni Richardson, 1904. 255 Ione thoracica (Montagu, 1808) . 256 Bopyridae Rafinesque, 1815 . 260 Pseudioninae Codreanu, 1967 . 260 Acrobelione Bourdon, 1981. 260 Acrobelione halimedae n. sp. 260 Key to females of species of Acrobelione Bourdon, 1981 . 262 Gyge Cornalia & Panceri, 1861. 262 Gyge branchialis Cornalia & Panceri, 1861 . 262 Gyge ovalis (Shiino, 1939) . 264 Ionella Bonnier, 1900 . -

Part I. an Annotated Checklist of Extant Brachyuran Crabs of the World
THE RAFFLES BULLETIN OF ZOOLOGY 2008 17: 1–286 Date of Publication: 31 Jan.2008 © National University of Singapore SYSTEMA BRACHYURORUM: PART I. AN ANNOTATED CHECKLIST OF EXTANT BRACHYURAN CRABS OF THE WORLD Peter K. L. Ng Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore, Kent Ridge, Singapore 119260, Republic of Singapore Email: [email protected] Danièle Guinot Muséum national d'Histoire naturelle, Département Milieux et peuplements aquatiques, 61 rue Buffon, 75005 Paris, France Email: [email protected] Peter J. F. Davie Queensland Museum, PO Box 3300, South Brisbane, Queensland, Australia Email: [email protected] ABSTRACT. – An annotated checklist of the extant brachyuran crabs of the world is presented for the first time. Over 10,500 names are treated including 6,793 valid species and subspecies (with 1,907 primary synonyms), 1,271 genera and subgenera (with 393 primary synonyms), 93 families and 38 superfamilies. Nomenclatural and taxonomic problems are reviewed in detail, and many resolved. Detailed notes and references are provided where necessary. The constitution of a large number of families and superfamilies is discussed in detail, with the positions of some taxa rearranged in an attempt to form a stable base for future taxonomic studies. This is the first time the nomenclature of any large group of decapod crustaceans has been examined in such detail. KEY WORDS. – Annotated checklist, crabs of the world, Brachyura, systematics, nomenclature. CONTENTS Preamble .................................................................................. 3 Family Cymonomidae .......................................... 32 Caveats and acknowledgements ............................................... 5 Family Phyllotymolinidae .................................... 32 Introduction .............................................................................. 6 Superfamily DROMIOIDEA ..................................... 33 The higher classification of the Brachyura ........................ -

Prawn Fauna (Crustacea: Decapoda) of India - an Annotated Checklist of the Penaeoid, Sergestoid, Stenopodid and Caridean Prawns
Available online at: www.mbai.org.in doi: 10.6024/jmbai.2012.54.1.01697-08 Prawn fauna (Crustacea: Decapoda) of India - An annotated checklist of the Penaeoid, Sergestoid, Stenopodid and Caridean prawns E. V. Radhakrishnan*1, V. D. Deshmukh2, G. Maheswarudu3, Jose Josileen 1, A. P. Dineshbabu4, K. K. Philipose5, P. T. Sarada6, S. Lakshmi Pillai1, K. N. Saleela7, Rekhadevi Chakraborty1, Gyanaranjan Dash8, C.K. Sajeev1, P. Thirumilu9, B. Sridhara4, Y Muniyappa4, A.D.Sawant2, Narayan G Vaidya5, R. Dias Johny2, J. B. Verma3, P.K.Baby1, C. Unnikrishnan7, 10 11 11 1 7 N. P. Ramachandran , A. Vairamani , A. Palanichamy , M. Radhakrishnan and B. Raju 1CMFRI HQ, Cochin, 2Mumbai RC of CMFRI, 3Visakhapatnam RC of CMFRI, 4Mangalore RC of CMFRI, 5Karwar RC of CMFRI, 6Tuticorin RC of CMFRI, 7Vizhinjam RC of CMFRI, 8Veraval RC of CMFRI, 9Madras RC of CMFRI, 10Calicut RC of CMFRI, 11Mandapam RC of CMFRI *Correspondence e-mail: [email protected] Received: 07 Sep 2011, Accepted: 15 Mar 2012, Published: 30 Apr 2012 Original Article Abstract Many penaeoid prawns are of considerable value for the fishing Introduction industry and aquaculture operations. The annual estimated average landing of prawns from the fishery in India was 3.98 The prawn fauna inhabiting the marine, estuarine and lakh tonnes (2008-10) of which 60% were contributed by freshwater ecosystems of India are diverse and fairly well penaeid prawns. An additional 1.5 lakh tonnes is produced from known. Significant contributions to systematics of marine aquaculture. During 2010-11, India exported US $ 2.8 billion worth marine products, of which shrimp contributed 3.09% in prawns of Indian region were that of Milne Edwards (1837), volume and 69.5% in value of the total export. -

(Marmara Sea) and Ecological Characteristics of Their Habitats
RESEARCH ARTICLE Eur J Biol 2017; 76(1): 20-5 Decapod Crustaceans in the Marmara Island (Marmara Sea) and Ecological Characteristics of Their Habitats Begum Ayfer, Husamettin Balkis, Aysegul Mulayim* Istanbul University, Faculty of Science, Department of Biology, Istanbul, Turkey Please cite this article as: Ayfer B, Balkis H, Mulayim A. Decapod Crustaceans in the Marmara Island (Marmara Sea) and Ecological Characteristics of Their Habitats. Eur J Biol 2017; 76(1): 20-5. ABSTRACT We have performed series of analyses to identify decapod crustaceans inhabiting the littoral zone of the Marmara Island and to study specific ecological characteristics of the habitat. Samples of decapod crustaceans species were collected from 12 stations (6 onshore, 6 offshore) on May 12-17, 2008 and November 17-22, 2008. A total of 17 species and 1199 specimens of decapod crustaceans were recorded. Eigth species (A. lacazei, N. norvegicus, P. bluteli, P. longimana, P. platycheles, D. pugilator, D. personata and L. vernalis) have been reported in the littoral zone of Marmara Island for the first time in this study. Also our study also sheds light on some ecological properties (temperature, salinity, dissolved oxygen) of the habitats of the species from the littoral zone of the Marmara Island. Keywords: Ecology, decapoda, crustacea, Marmara Island, The Sea of Marmara INTRODUCTION The first study at the island was carried out by Ostrou- moff (3,4) followed by studies by Okuş (5), Yüksek (6) The Archipelago in the Sea of Marmara consisting of and Balkıs (7). small and large islands located southwest of the Sea of Marmara and the northwest of the Kapıdağ Peninsula MATERIALS AND METHODS are referred to as the Islands of Marmara. -

How to Become a Crab: Phenotypic Constraints on a Recurring Body Plan
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 25 December 2020 doi:10.20944/preprints202012.0664.v1 How to become a crab: Phenotypic constraints on a recurring body plan Joanna M. Wolfe1*, Javier Luque1,2,3, Heather D. Bracken-Grissom4 1 Museum of Comparative Zoology and Department of Organismic & Evolutionary Biology, Harvard University, 26 Oxford St, Cambridge, MA 02138, USA 2 Smithsonian Tropical Research Institute, Balboa–Ancon, 0843–03092, Panama, Panama 3 Department of Earth and Planetary Sciences, Yale University, New Haven, CT 06520-8109, USA 4 Institute of Environment and Department of Biological Sciences, Florida International University, Biscayne Bay Campus, 3000 NE 151 Street, North Miami, FL 33181, USA * E-mail: [email protected] Summary: A fundamental question in biology is whether phenotypes can be predicted by ecological or genomic rules. For over 140 years, convergent evolution of the crab-like body plan (with a wide and flattened shape, and a bent abdomen) at least five times in decapod crustaceans has been known as ‘carcinization’. The repeated loss of this body plan has been identified as ‘decarcinization’. We offer phylogenetic strategies to include poorly known groups, and direct evidence from fossils, that will resolve the pattern of crab evolution and the degree of phenotypic variation within crabs. Proposed ecological advantages of the crab body are summarized into a hypothesis of phenotypic integration suggesting correlated evolution of the carapace shape and abdomen. Our premise provides fertile ground for future studies of the genomic and developmental basis, and the predictability, of the crab-like body form. Keywords: Crustacea, Anomura, Brachyura, Carcinization, Phylogeny, Convergent evolution, Morphological integration 1 © 2020 by the author(s).