Thesis for Word XP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome of Phaeocystis Globosa Virus Pgv-16T Highlights the Common Ancestry of the Largest Known DNA Viruses Infecting Eukaryotes
Genome of Phaeocystis globosa virus PgV-16T highlights the common ancestry of the largest known DNA viruses infecting eukaryotes Sebastien Santinia, Sandra Jeudya, Julia Bartolia, Olivier Poirota, Magali Lescota, Chantal Abergela, Valérie Barbeb, K. Eric Wommackc, Anna A. M. Noordeloosd, Corina P. D. Brussaardd,e,1, and Jean-Michel Claveriea,f,1 aStructural and Genomic Information Laboratory, Unité Mixte de Recherche 7256, Centre National de la Recherche Scientifique, Aix-Marseille Université, 13288 Marseille Cedex 9, France; bCommissariat à l’Energie Atomique–Institut de Génomique, 91057 Evry Cedex, France; cDepartment of Plant and Soil Sciences, University of Delaware, Newark, DE 19711; dDepartment of Biological Oceanography, Royal Netherlands Institute for Sea Research, NL-1790 AB Den Burg (Texel), The Netherlands; eAquatic Microbiology, Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, Amsterdam, The Netherlands; and fService de Santé Publique et d’Information Médicale, Hôpital de la Timone, Assistance Publique–Hôpitaux de Marseille, FR-13385 Marseille, France Edited by James L. Van Etten, University of Nebraska, Lincoln, NE, and approved May 1, 2013 (received for review February 22, 2013) Large dsDNA viruses are involved in the population control of many viruses: 730 kb and 1.28 Mb for CroV and Megavirus chilensis, globally distributed species of eukaryotic phytoplankton and have respectively. Other studies, targeting virus-specific genes [e.g., a prominent role in bloom termination. The genus Phaeocystis (Hap- DNA polymerase B (8) or capsid proteins (9)] have suggested tophyta, Prymnesiophyceae) includes several high-biomass-forming a close phylogenetic relationship between Mimivirus and several phytoplankton species, such as Phaeocystis globosa, the blooms of giant dsDNA viruses infecting various unicellular algae such as which occur mostly in the coastal zone of the North Atlantic and the Pyramimonas orientalis (Chlorophyta, Prasinophyceae), Phaeocys- North Sea. -

Biological Phosphorylation of an Unnatural Base Pair (UBP) Using a Drosophila Melanogaster Deoxynucleoside Kinase (Dmdnk) Mutant
RESEARCH ARTICLE Biological phosphorylation of an Unnatural Base Pair (UBP) using a Drosophila melanogaster deoxynucleoside kinase (DmdNK) mutant Fei Chen1,2,3☯*, Yuan Zhang4☯, Ashley B. Daugherty5, Zunyi Yang3, Ryan Shaw3, Mengxing Dong1,2, Stefan Lutz5, Steven A. Benner3* a1111111111 1 CAS Key Laboratory of Genome Sciences & Information, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing, China, 2 University of Chinese Academy of Sciences, Beijing, China, 3 Foundation for a1111111111 Applied Molecular Evolution (FfAME), Alachua, Florida, United States of America, 4 College of Chemistry, a1111111111 Beijing Normal University, Beijing, China, 5 Department of Chemistry, Emory University School of Medicine, a1111111111 Atlanta, Georgia, United States of America a1111111111 ☯ These authors contributed equally to this work. * [email protected] (FC); [email protected] (SAB) OPEN ACCESS Abstract Citation: Chen F, Zhang Y, Daugherty AB, Yang Z, Shaw R, Dong M, et al. (2017) Biological One research goal for unnatural base pair (UBP) is to replicate, transcribe and translate phosphorylation of an Unnatural Base Pair (UBP) them in vivo. Accordingly, the corresponding unnatural nucleoside triphosphates must be using a Drosophila melanogaster deoxynucleoside available at sufficient concentrations within the cell. To achieve this goal, the unnatural kinase (DmdNK) mutant. PLoS ONE 12(3): nucleoside analogues must be phosphorylated to the corresponding nucleoside triphos- e0174163. https://doi.org/10.1371/journal. pone.0174163 phates by a cascade of three kinases. The first step is the monophosphorylation of unnatural deoxynucleoside catalyzed by deoxynucleoside kinases (dNK), which is generally consid- Editor: Giovanni Maga, Istituto di Genetica Molecolare, ITALY ered the rate limiting step because of the high specificity of dNKs. -

Deoxynucleoside Kinases Development in Vitro by Inhibition
Restoration of Adenosine Deaminase-Deficient Human Thymocyte Development In Vitro by Inhibition of Deoxynucleoside Kinases This information is current as of September 26, 2021. Michelle L. Joachims, Patrick A. Marble, Aletha B. Laurent, Peter Pastuszko, Marco Paliotta, Michael R. Blackburn and Linda F. Thompson J Immunol 2008; 181:8153-8161; ; doi: 10.4049/jimmunol.181.11.8153 Downloaded from http://www.jimmunol.org/content/181/11/8153 References This article cites 54 articles, 20 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/181/11/8153.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 26, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Restoration of Adenosine Deaminase-Deficient Human Thymocyte Development In Vitro by Inhibition of Deoxynucleoside Kinases1,2 Michelle L. Joachims,* Patrick A. Marble,* Aletha B. Laurent,* Peter Pastuszko,3† Marco Paliotta,† Michael R. -

Conserved Phosphoryl Transfer Mechanisms Within Kinase Families
Kenyon et al. BMC Research Notes 2012, 5:131 http://www.biomedcentral.com/1756-0500/5/131 RESEARCHARTICLE Open Access Conserved phosphoryl transfer mechanisms within kinase families and the role of the C8 proton of ATP in the activation of phosphoryl transfer Colin P Kenyon*, Robyn L Roth, Chris W van der Westhuyzen and Christopher J Parkinson Abstract Background: The kinome is made up of a large number of functionally diverse enzymes, with the classification indicating very little about the extent of the conserved kinetic mechanisms associated with phosphoryl transfer. It has been demonstrated that C8-H of ATP plays a critical role in the activity of a range of kinase and synthetase enzymes. Results: A number of conserved mechanisms within the prescribed kinase fold families have been identified directly utilizing the C8-H of ATP in the initiation of phosphoryl transfer. These mechanisms are based on structurally conserved amino acid residues that are within hydrogen bonding distance of a co-crystallized nucleotide. On the basis of these conserved mechanisms, the role of the nucleotide C8-H in initiating the formation of a pentavalent intermediate between the g-phosphate of the ATP and the substrate nucleophile is defined. All reactions can be clustered into two mechanisms by which the C8-H is induced to be labile via the coordination of a backbone carbonyl to C6-NH2 of the adenyl moiety, namely a “push” mechanism, and a “pull” mechanism, based on the protonation of N7. Associated with the “push” mechanism and “pull” mechanisms are a series of proton transfer cascades, initiated from C8-H, via the tri-phosphate backbone, culminating in the formation of the pentavalent transition state between the g-phosphate of the ATP and the substrate nucleophile. -

Development of Gene Therapy in Association with Clinically Used
Cancer Gene Therapy (2009) 16, 541–550 r 2009 Nature Publishing Group All rights reserved 0929-1903/09 $32.00 www.nature.com/cgt REVIEW Development of gene therapy in association with clinically used cytotoxic deoxynucleoside analogues CHe´brard1,2, C Dumontet1,2 and LP Jordheim1,2 1Universite´ de Lyon, Lyon, France and 2INSERM U590, Lyon, France The clinical use of cytotoxic deoxynucleoside analogues is often limited by resistance mechanisms due to enzymatic deficiency, or high toxicity in nontumor tissues. To improve the use of these drugs, gene therapy approaches have been proposed and studied, associating clinically used deoxynucleoside analogues such as araC and gemcitabine and suicide genes or myeloprotective genes. In this review, we provide an update of recent results in this area, with particular emphasis on human deoxycytidine kinase, the deoxyribonucleoside kinase from Drosophila melanogaster, purine nucleoside phosphorylase from Escherichia coli, and human cytidine deaminase. Data from literature clearly show the feasibility of these systems, and clinical trials are warranted to conclude on their use in the treatment of cancer patients. Cancer Gene Therapy (2009) 16, 541–550; doi:10.1038/cgt.2009.25; published online 3 April 2009 Keywords: suicide gene; deoxynucleoside analogues; dCK; Dm-dNK; cytidine deaminase; PNP Introduction specifically activated and cytotoxic in transfected tumor cells. Myeloprotective gene therapy is based on the Deoxynucleoside analogues are widely used in the introduction of a gene conferring resistance to chemother- treatment of hematological malignancies and solid apeutic agents, into normal blood stem cells. When these tumors. A limiting factor in the use of these agents is cells are reintroduced into the bone marrow before the appearance of resistance or the presence of non- chemotherapy the hematological toxicity will be decreased responding cancer cells.1 Several approaches are currently because the gene product will inactivate the drug. -

Overview of the Synthesis of Nucleoside Phosphates and Polyphosphates 13.1.6
Overview of the Synthesis of Nucleoside UNIT 13.1 Phosphates and Polyphosphates Phosphorylated nucleosides play a domi- ity to the synthesis. Side reactions can occur, nant role in biochemistry. Primary metabolism, such as depurination of the nucleoside, phos- DNA replication and repair, RNA synthesis, phorylation of the nucleobase, as well as chemi- protein synthesis, signal transduction, polysac- cal alteration of nucleobase analogs. Due to charide biosynthesis, and enzyme regulation their intrinsic reactivity, the synthesis of phos- are just a handful of processes involving these phoanhydride bonds is also synthetically chal- molecules. Literally thousands of enzymes use lenging. Phosphate anhydrides are phosphory- these compounds as substrates and/or regula- lating reagents that are readily degraded under tors. The need to obtain such compounds in acidic conditions. Finally, purification of syn- both labeled and unlabeled forms, as well as a thetic nucleotides can be problematic. Ionic burgeoning need for analogs, has driven the reagents, starting materials, and mixtures of development of a myriad of chemical and en- regioisomers (2′-, 3′-, 5′-phosphates) can be zymatic synthetic approaches. As chemical en- particularly difficult to separate from the de- tities, few molecules possess the wide array of sired product. densely packed functionality present in phos- In spite of the many potential difficulties phorylated nucleosides. This poses a formida- associated with nucleoside phosphorylation ble challenge to the synthetic chemist, one that and polyphosphorylation, a certain amount of has not yet been fully overcome. This overview success has been achieved in these areas. Given will address some common methods (synthetic the wealth of phosphorylating reagents avail- and enzymatic) used to construct phosphory- able, simple phosphorylation of nucleosides at lated nucleosides. -

Phosphorylation of Acyclovir in Vitro in Activated Burkitt Somatic Cell Hybrids ALOK K
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, July 1983, p. 10-14 Vol. 24, No. 1 0064804/83/070010-05$02.00/0 Copyright 0 1983, American Society for Microbiology Phosphorylation of Acyclovir In Vitro in Activated Burkitt Somatic Cell Hybrids ALOK K. DATTAl 2* AND JOSEPH S. PAGANO3'4 Cancer Research Centerl* and Departments ofBiochemistry and Nutrition,2 Medicine,3 and Bacteriology and Immunology,' School ofMedicine, University ofNorth Carolina, Chapel Hill, North Carolina 27514 Received 10 December 1982/Accepted 14 April 1983 Acyclovir [9-(2-hydroxyethoxymethyl)guanineI (ACV), a potent antiviral com- pound, was phosphorylated to the same extent by extracts from untreated and iododeoxyuridine-treated Epstein-Barr virus-containing latent D98/HR-1 somatic hybrid cells. ATP was the preferred phosphate donor over other nucleoside triphosphates. The cytosol extract from D98/HR-1 cells effected optimum phos- phorylation of thymidine at pH 8.0, whereas ACV was phosphorylated equally well over a wide pH range. Electrophoretic analysis of thymidine kinase-, deoxycytidine kinase-, and ACV-phosphorylating activities from both untreated and iododeQxyuridine-treated cell extracts displayed identical properties. A small part (5 to 10%o) of the loaded ACV-phosphorylating activity seemed to migrate with the deoxycytidine kinase activity from cytosol. dTTP and dCTP, at relatively high concentrations, partially inhibited ACV-phosphorylating activity. The results suggest that Epstein-Barr virus does not code for its own thymidine kinase and that phosphorylation -

12) United States Patent (10
US007635572B2 (12) UnitedO States Patent (10) Patent No.: US 7,635,572 B2 Zhou et al. (45) Date of Patent: Dec. 22, 2009 (54) METHODS FOR CONDUCTING ASSAYS FOR 5,506,121 A 4/1996 Skerra et al. ENZYME ACTIVITY ON PROTEIN 5,510,270 A 4/1996 Fodor et al. MICROARRAYS 5,512,492 A 4/1996 Herron et al. 5,516,635 A 5/1996 Ekins et al. (75) Inventors: Fang X. Zhou, New Haven, CT (US); 5,532,128 A 7/1996 Eggers Barry Schweitzer, Cheshire, CT (US) 5,538,897 A 7/1996 Yates, III et al. s s 5,541,070 A 7/1996 Kauvar (73) Assignee: Life Technologies Corporation, .. S.E. al Carlsbad, CA (US) 5,585,069 A 12/1996 Zanzucchi et al. 5,585,639 A 12/1996 Dorsel et al. (*) Notice: Subject to any disclaimer, the term of this 5,593,838 A 1/1997 Zanzucchi et al. patent is extended or adjusted under 35 5,605,662 A 2f1997 Heller et al. U.S.C. 154(b) by 0 days. 5,620,850 A 4/1997 Bamdad et al. 5,624,711 A 4/1997 Sundberg et al. (21) Appl. No.: 10/865,431 5,627,369 A 5/1997 Vestal et al. 5,629,213 A 5/1997 Kornguth et al. (22) Filed: Jun. 9, 2004 (Continued) (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2005/O118665 A1 Jun. 2, 2005 EP 596421 10, 1993 EP 0619321 12/1994 (51) Int. Cl. EP O664452 7, 1995 CI2O 1/50 (2006.01) EP O818467 1, 1998 (52) U.S. -

Genomic Recombination Leading to Decreased Virulence of Group B Streptococcus in a Mouse Model of Adult Invasive Disease
Pathogens 2016, 5, 54 S1 of S15 Supplementary Materials: Genomic Recombination Leading to Decreased Virulence of Group B Streptococcus in a Mouse Model of Adult Invasive Disease Sarah Teatero, Paul Lemire, Ken Dewar, Jessica Wasserscheid, Cynthia Calzas, Gustavo V. Mallo, Aimin Li, Taryn B.T. Athey, Mariela Segura and Nahuel Fittipaldi Figure S1. Genome atlas of the complete genome of NGBS357 (ST1). Depicted data from innermost to outermost circles represent genome size in Mbp (circle 1), percent G+C content (Circle 2), GC skew, where (G-C)/(G+C) is averaged over a moving window of 10,000 bp, with excess C shown in green and purple, respectively (circle 3). Circle 4 shows annotated coding sequences (CDSs) on the forward strand (dark blue), while circle 5 shows reverse-strand encoded CDSs (light blue). Reference landmarks (circle 6): mobile genetic elements, black; RNA, green; MLST genes, light blue; bibA (hvgA homologue) and other genes of interest, orange. Pathogens 2016, 5, 54 S2 of S15 Table S1. Whole Genome Sequencing statistics for genome closure. Accession No. of Reads Ave. Length of Coverage of Final No. of Reads Length of Reads Coverage of Final Genome Strain Number >3 kb (PacBio) (bp) Reads (PacBio) (bp) Assemblies (PacBio) (Illumina) (Illumina) Assemblies (Illumina) Size NGBS375 CP012503 62,706 6087 176 2,250,144 150 155 2,172,875 NGBS357 CP012504 45,296 8119 166 2,146,206 101 98 2,208,841 a a Size of sequenced contig. Does not include repetitive region. Pathogens 2016, 5, 54 S3 of S15 Table S2. Gene content in recombined region of NGBS375 (ST297). -

Development of Capillary Electrophoresis-Based Assays For
DDeevveellooppmmeenntt ooff ccaappiillllaarryy eelleeccttrroopphhoorreessiiss-- bbaasseedd aassssaayyss ffoorr nnuucclleeoossiiddee aanndd nnuucclleeoottiiddee mmeettaabboolliizziinngg eennzzyymmeess Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn Vorgelegt von Jamshed Iqbal aus Sialkot, Pakistan Bonn 2005 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn Diese Dissertation ist auf dem Hochschulschriftenserver der ULB Bonn http://hss.ulb.uni- bonn.de/diss_online elektronisch publiziert 1. Referent: Prof. Dr. Christa E. Müller 2. Referent: Priv.-Doz. Dr. Michael Neugebauer Tag der Promotion: Die vorliegende Arbeit wurde in der Zeit von November 2001 bis October 2005 am Pharmazeutischen Institut der Universität Bonn in Poppelsdorf unter Leitung von Frau Prof. Dr. Christa E. Müller durchgeführt. Contents Page 1. Capillary electrophoresis 1 1.1. Electroosmotic flow 2 1.2. Capillary zone electrophoresis 4 1.3. Micellar electrokinetic capillary chromatography 5 1.4. Coating of the fused-silica capillary surface 6 1.4.1. Dynamic coating 7 1.4.2. Permanent coating 7 2. Development of capillary electrophoresis methods for high-resolution separation and quantitation of nucleosides and nucleotides 8 2.1. Separation and quantitation of adenosine, AMP, ADP, ATP and UMP by capillary electrophoresis using micellar electrokinetic capillary chromatography 8 2.1.1. Introduction 8 2.1.2. Experimental 10 2.1.2.1. Materials and methods 10 2.1.2.1.1. Capillary electrophoresis instrumentation 10 2.1.2.1.2. Chemicals 10 2.1.2.1.3. Preparation of standard solutions 10 2.2. Results and discussion 11 2.2.1. Separation of nucleotides 11 2.2.2. Development of a capillary electrophoresis method for the determination of nucleotides 12 2.2.2.1. -
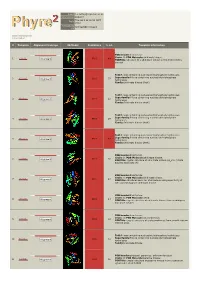
Phyre 2 Results for P0A6D7
Email [email protected] Description P0A6D7 Thu Jan 5 11:02:54 GMT Date 2012 Unique Job 0244e86604702aa1 ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:transferase Chain: B: PDB Molecule:shikimate kinase; 1 c3trfB_ 99.9 44 Alignment PDBTitle: structure of a shikimate kinase (arok) from coxiella burnetii Fold:P-loop containing nucleoside triphosphate hydrolases Superfamily:P-loop containing nucleoside triphosphate 2 d2iyva1 99.9 37 Alignment hydrolases Family:Shikimate kinase (AroK) Fold:P-loop containing nucleoside triphosphate hydrolases Superfamily:P-loop containing nucleoside triphosphate 3 d1e6ca_ 99.9 32 Alignment hydrolases Family:Shikimate kinase (AroK) Fold:P-loop containing nucleoside triphosphate hydrolases Superfamily:P-loop containing nucleoside triphosphate 4 d1viaa_ 99.9 29 Alignment hydrolases Family:Shikimate kinase (AroK) Fold:P-loop containing nucleoside triphosphate hydrolases Superfamily:P-loop containing nucleoside triphosphate 5 d1kaga_ 99.9 97 Alignment hydrolases Family:Shikimate kinase (AroK) PDB header:transferase Chain: D: PDB Molecule:shikimate kinase; 6 c2pt5D_ 99.9 36 Alignment PDBTitle: crystal structure of shikimate kinase (aq_2177) from aquifex aeolicus2 vf5 PDB header:transferase Chain: A: PDB Molecule:shikimate kinase; 7 c1zuiA_ 99.9 29 Alignment PDBTitle: structural basis for shikimate-binding specificity of helicobacter2 pylori shikimate kinase PDB header:transferase Chain: B: PDB Molecule:atsk2; 8 c3nwjB_ 99.9 24 Alignment -

Modular Enzymatic Cascade Synthesis of Nucleotides Using a (D)ATP Regeneration System
fbioe-08-00854 October 21, 2020 Time: 16:51 # 1 ORIGINAL RESEARCH published: 06 August 2020 doi: 10.3389/fbioe.2020.00854 Modular Enzymatic Cascade Synthesis of Nucleotides Using a (d)ATP Regeneration System Maryke Fehlau1,2, Felix Kaspar1,2, Katja F. Hellendahl1, Julia Schollmeyer1,2, Peter Neubauer1 and Anke Wagner1,2* 1 Chair of Bioprocess Engineering, Institute of Biotechnology, Faculty III Process Sciences, Technische Universität Berlin, Berlin, Germany, 2 BioNukleo GmbH, Berlin, Germany Nucleoside-5’-triphosphates (NTPs) and their analogs are building blocks of DNA and are important compounds in both pharmaceutical and molecular biology applications. Currently, commercially available base or sugar modified NTPs are mainly synthesized chemically. Since the chemical production of NTPs is time-consuming and generally inefficient, alternative approaches are under development. Here we present a simple, efficient and generalizable enzymatic synthesis method for the conversion of nucleosides Edited by: to NTPs. Our one-pot method is modular, applicable to a wide range of natural Simon J. Moore, University of Kent, United Kingdom and modified nucleotide products and accesses NTPs directly from cheap nucleoside Reviewed by: precursors. Nucleoside kinases, nucleoside monophosphate (NMP) kinases and a Lothar Elling, nucleoside diphosphate (NDP) kinase were applied as biocatalysts. Enzymes with RWTH Aachen University, Germany different substrate specificities were combined to produce derivatives of adenosine Zongbao K. Zhao, Dalian Institute of Chemical Physics and cytidine triphosphate with conversions of 4 to 26%. The implementation of a (CAS), China (deoxy)ATP recycling system resulted in a significant increase in the conversion to all NTP *Correspondence: products, furnishing 4 different NTPs in quantitative conversion.