The Hormonal Regulation of Pyloric Sphincter Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fecal Incontinence/Anal Incontinence
Fecal Incontinence/Anal Incontinence What are Fecal incontinence/ Anal Incontinence? Fecal incontinence is inability to control solid or liquid stool. Anal incontinence is the inability to control gas and mucous in addition to the inability to control stool. The symptoms range from mild release of gas to a complete loss of control. It is a common problem affecting 1 out of 13 women under the age of 60 and 1 out of 7 women over the age of 60. Men can also be have this condition. Anal incontinence is a distressing condition that can interfere with the ability to work, do daily activities and enjoy social events. Even though anal incontinence is a common condition, people are uncomfortable discussing this problem with family, friends, or doctors. They often suffer in silence, not knowing that help is available. Normal anatomy The anal sphincters and puborectalis are the primary muscles responsible for continence. There are two sphincters: the internal anal sphincter, and the external anal sphincter. The internal sphincter is responsible for 85% of the resting muscle tone and is involuntary. This means, that you do not have control over this muscle. The external sphincter is responsible for 15% of your muscle tone and is voluntary, meaning you have control over it. Squeezing the puborectalis muscle and external anal sphincter together closes the anal canal. Squeezing these muscles can help prevent leakage. Puborectalis Muscle Internal Sphincter External Sphincter Michigan Bowel Control Program - 1 - Causes There are many causes of anal incontinence. They include: Injury or weakness of the sphincter muscles. Injury or weakening of one of both of the sphincter muscles is the most common cause of anal incontinence. -

Mouth Esophagus Stomach Rectum and Anus Large Intestine Small
1 Liver The liver produces bile, which aids in digestion of fats through a dissolving process known as emulsification. In this process, bile secreted into the small intestine 4 combines with large drops of liquid fat to form Healthy tiny molecular-sized spheres. Within these spheres (micelles), pancreatic enzymes can break down fat (triglycerides) into free fatty acids. Pancreas Digestion The pancreas not only regulates blood glucose 2 levels through production of insulin, but it also manufactures enzymes necessary to break complex The digestive system consists of a long tube (alimen- 5 carbohydrates down into simple sugars (sucrases), tary canal) that varies in shape and purpose as it winds proteins into individual amino acids (proteases), and its way through the body from the mouth to the anus fats into free fatty acids (lipase). These enzymes are (see diagram). The size and shape of the digestive tract secreted into the small intestine. varies in each individual (e.g., age, size, gender, and disease state). The upper part of the GI tract includes the mouth, throat (pharynx), esophagus, and stomach. The lower Gallbladder part includes the small intestine, large intestine, The gallbladder stores bile produced in the liver appendix, and rectum. While not part of the alimentary 6 and releases it into the duodenum in varying canal, the liver, pancreas, and gallbladder are all organs concentrations. that are vital to healthy digestion. 3 Small Intestine Mouth Within the small intestine, millions of tiny finger-like When food enters the mouth, chewing breaks it 4 protrusions called villi, which are covered in hair-like down and mixes it with saliva, thus beginning the first 5 protrusions called microvilli, aid in absorption of of many steps in the digestive process. -

Sphincter of Oddi: ERCP Plus Sphincterotomy – Yes Or No
Sphincter of Oddi: ERCP Plus Sphincterotomy – Yes or No Note: For debate purposes, the pro and con positions for patient management will be taken by the invited authors. However, actual decisions regarding patient care must involve discussion of the risks and benefits of each treatment considered. Case Presentation – Case developed by Ihab I. El Hajj, MD, MPH, Indiana University, Indianapolis, IN A 57-year-old Caucasian female with history of smoking and COPD, was in her usual state of health until two years ago, when she experienced recurrent “attacks” of right upper quadrant pain, nausea and occasional vomiting, suggestive of biliary colic. The patient was evaluated by her primary care physician and initial work- up, which included basic blood work, liver chemistries and transabdominal ultrasound, were negative. The patient responded partially to prn Zofran and omeprazole 40 mg once then twice daily. With the persistence of her symptoms, the patient was referred to a gastroenterologist. Esophagogastroduodenoscopy (EGD) with gastric biopsies revealed chronic inactive gastritis without Helicobacter pylori. HIDA scan suggested biliary dyskinesia with an ejection fraction of 22%. An elective laparoscopic cholecystectomy was performed. An intra- operative cholangiogram showed no filling defect in the common bile duct (CBD) and pathology demonstrated chronic cholecystitis with no gallstones. The patient was symptom-free for six months after surgery. She subsequently developed vague upper abdominal pain, intermittent nausea and irregular bowel movements. Labs, colonoscopy and repeat EGD were ASGE Leading Edge — Volume 4, No. 4 © American Society for Gastrointestinal Endoscopy normal. The patient was treated for suspected irritable bowel syndrome. She failed several medications including hyoscyamine, dicyclomine, amitriptyline, sucralfate, and GI cocktail. -

Progress Report Anal Continence
Gut: first published as 10.1136/gut.12.10.844 on 1 October 1971. Downloaded from Gut, 1971, 12, 844-852 Progress report Anal continence Anal continence depends on an adaptable barrier formed at the ano-rectal junction and in the anal canal by a combination of forces. These are due in part to the configuration of the region and in part to the action of muscles. The forces are activated in response to sensory information obtained from the rectum and the anal canal. In order to understand some of the concepts of the mechanism of anal continence, some of the features of the anatomy and physiology of the region will be discussed. Anatomy (Fig. 1) The lumen of the rectum terminates at the pelvic floor and is continued, downwards and posteriorly, as the anal canal, passing through the levator ani muscle sheet and surrounded by the internal and external anal sphincters. The anal canal is 2.5 to 5 cm in length and 3 cm in diameter when distended. The axis of the rectum forms almost a right angle (average 820) with the axis of the anal canal. It has been established by radiological studies that the anal canal is an antero-posterior slit in the resting state.' The former concept of http://gut.bmj.com/ the anal canal being surrounded successively craniocaudally by the internal anal sphincter and then the external anal sphincter has been replaced by the knowledge that the two muscles overlap to a considerable extent with the external sphincter wrapped round the internal sphincter2'3. -

The Gallbladder
The Gallbladder Anatomy of the gallbladder Location: Right cranial abdominal quadrant. In the gallbladder fossa of the liver. o Between the quadrate and right medial liver lobes. Macroscopic: Pear-shaped organ Fundus, body and neck. o Neck attaches, via a short cystic duct, to the common bile duct. Opens into the duodenum via sphincter of Oddi at the major duodenal papilla. Found on the mesenteric margin of orad duodenum. o 3-6 cm aboral to pylorus. 1-2cm of distal common bile duct runs intramural. Species differences: Dogs: o Common bile duct enters at major duodenal papilla. Adjacent to pancreatic duct (no confluence prior to entrance). o Accessory pancreatic duct enters at minor duodenal papilla. ± 2 cm aboral to major duodenal papilla. MAJOR conduit for pancreatic secretions. Cats: o Common bile duct and pancreatic duct converge before opening at major duodenal papilla. Thus, any surgical procedure that affects the major duodenal papilla can affect the exocrine pancreatic secretions in cats. o Accessory pancreatic duct only seen in 20% of cats. 1 Gallbladder wall: 5 histologically distinct layers. From innermost these include: o Epithelium, o Submucosa (consisting of the lamina propria and tunica submucosa), o Tunica muscularis externa, o Tunica serosa (outermost layer covers gallbladder facing away from the liver), o Tunica adventitia (outermost layer covers gallbladder facing towards the liver). Blood supply: Solely by the cystic artery (derived from the left branch of the hepatic artery). o Susceptible to ischaemic necrosis should its vascular supply become compromised. Function: Storage reservoir for bile o Concentrated (up to 10-fold), acidified (through epithelial acid secretions) and modified (by the addition of mucin and immunoglobulins) before being released into the gastrointestinal tract at the major duodenal. -

Internal Anal Sphincter
Arch Dis Child: first published as 10.1136/adc.43.231.569 on 1 October 1968. Downloaded from Arch. Dis. Childh., 1968, 43, 569. Internal Anal Sphincter Observations on Development and Mechanism of Inhibitory Responses in Premature Infants and Children with Hirschsprung's Disease E. R. HOWARD and H. H. NIXON From The Hospitalfor Sick Children, Great Ormond Street, London W.C.1 The relative importance of the internal and obstruction to constipation alone. During this external sphincters to the maintenance of tone in study physiological abnormalities were observed in the anal canal has been shown in previous studies of the reflexes of premature infants, which showed anal physiology (Gaston, 1948; Schuster et al., 1965; similarities to those seen in patients with Hirsch- Duthie and Watts, 1965). sprung's disease. On repeated examinations over The external sphincter is a striated muscle, but several days, however, the physiological responses shows continuous activity on electromyography. were found to change until normal reflexes were Inhibition and stimulation is mediated by spinal eventually established. cord reflexes, through the pudendal nerves and In order to help determine the nervous pathway sacral segments of the spinal cord (Floyd and Walls, through which the reflexes of the internal sphincter copyright. 1953; Porter, 1961). Voluntary control is possible are mediated, we have examined normal bowel over this part ofthe anal sphincter. and aganglionic bowel from cases of Hirschsprung's The internal sphincter is made up of smooth disease by pharmacological and histochemical muscle fibres, continuous with the muscle layers of methods. the rectal wall, and under resting conditions pro- vides most of the tone of the anal canal (Duthie and Physiological Study Watts, 1965). -

Accessory Pancreatic Duct Patterns and Their Clinical Implications Anatomy Section
DOI: 10.7860/JCDR/2015/11539.5660 Original Article Accessory Pancreatic Duct Patterns and Their Clinical Implications Anatomy Section LOKADOLALU CHANDRACHARYA PRASANNA1, KV RAJAGOPAL2, HUBAN R THOMAS3, KUMAR MR BHAT4 ABSTRACT embryonic type of duct pattern was seen in 5% specimens. Context and Objective: Accessory pancreatic duct (APD) 75% of long type ducts showed positive patency with eosin designed to reduce the pressure of major pancreatic duct dye, followed by ansa type (44.4%), and least patency was by forming a secondary drainage channel. Few studies have found in short type (22.2%). With regard to the patency of the mentioned the variant types of accessory ducts and their mode accessory pancreatic ducts towards their termination, we found of formation, some of these have a clear clinical significance. 52.5% of the accessory ducts and 5% of the embryonic type Present study is aimed to evaluate the possible variations in the pancreatic ducts were patent and in 42.5% of the specimen APD and its terminations. the ducts were obliterated. In 85% of specimens the minor duodenal papillae was anterosuperior to the major papilla and Materials and Methods: Forty formalin fixed adult human superior to the major papillae in 10% of the cases, and in 5% pancreas with duodenum in situ specimens were studied by minor papillae was absent. The average distance between the injecting 1% aqueous eosin, followed by piece meal dissection two papillae was 2.35 cm. of the head of the pancreas from posterior surface. Formation, tributaries, relations, and the termination of the accessory Conclusion: The knowledge of the complex anatomical relations pancreatic duct were noted and photographed. -
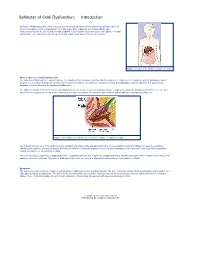
Sphincter of Oddi Dysfunction: Introduction
Sphincter of Oddi Dysfunction: Introduction Sphincter of Oddi dysfunction refers to structural or functional disorders involving the biliary sphincter that may result in impedance of bile and pancreatic juice flow. Up to 20% of patients with continued pain after cholecystectomy and 10–20% of patients with idiopathic recurrent pancreatitis may suffer from sphincter of Oddi dysfunction. This condition is more prevalent among middle-aged women for unclear reasons Figure 1. Location of the sphincter of Oddi in the body. What is Sphincter of Oddi Dysfunction? The sphincter of Oddi has three major functions: 1) regulation of bile and pancreatic flow into the duodenum, 2) diversion of hepatic bile into the gallbladder, and 3) the prevention of reflux of duodenal contents into the pancreaticobiliary tract. With the ingestion of a meal, the gallbladder contracts and there is a simultaneous decrease in the resistance in the sphincter of Oddi zone. The sphincter of Oddi consists of circular and longitudinal smooth muscle fibers surrounding a variable length of the distal bile and pancreatic duct. There are three discrete areas of muscle thickness, or mini sphincters: the sphincter papillae, the sphincter pancreaticus, and the sphincter choledochus (Figure 2). Figure 2. Mini sphincters, or discrete areas of muscle, comprise the sphincter of Oddi. The major physiologic role of the sphincter is the regulation of the flow of bile and pancreatic juice. Cholecystokinin (CCK) and nitrates decrease the resistance offered by the sphincter. Laboratory studies observing the effects of numerous peptides, hormones, and medications on the sphincter have suggested a multifactor control mechanism for the sphincter of Oddi. -

Chronic Pancreatitis: Introduction
Chronic Pancreatitis: Introduction Authors: Anthony N. Kalloo, MD; Lynn Norwitz, BS; Charles J. Yeo, MD Chronic pancreatitis is a relatively rare disorder occurring in about 20 per 100,000 population. The disease is progressive with persistent inflammation leading to damage and/or destruction of the pancreas . Endocrine and exocrine functional impairment results from the irreversible pancreatic injury. The pancreas is located deep in the retroperitoneal space of the upper part of the abdomen (Figure 1). It is almost completely covered by the stomach and duodenum . This elongated gland (12–20 cm in the adult) has a lobe-like structure. Variation in shape and exact body location is common. In most people, the larger part of the gland's head is located to the right of the spine or directly over the spinal column and extends to the spleen . The pancreas has both exocrine and endocrine functions. In its exocrine capacity, the acinar cells produce digestive juices, which are secreted into the intestine and are essential in the breakdown and metabolism of proteins, fats and carbohydrates. In its endocrine function capacity, the pancreas also produces insulin and glucagon , which are secreted into the blood to regulate glucose levels. Figure 1. Location of the pancreas in the body. What is Chronic Pancreatitis? Chronic pancreatitis is characterized by inflammatory changes of the pancreas involving some or all of the following: fibrosis, calcification, pancreatic ductal inflammation, and pancreatic stone formation (Figure 2). Although autopsies indicate that there is a 0.5–5% incidence of pancreatitis, the true prevalence is unknown. In recent years, there have been several attempts to classify chronic pancreatitis, but these have met with difficulty for several reasons. -

Normal Gastrointestinal Motility and Function Esophagus
Normal Gastrointestinal Motility and Function "Motility" is an unfamiliar word to many people; it is used primarily to describe the contraction of the muscles in the gastrointestinal tract. Because the gastrointestinal tract is a circular tube, when these muscles contract, they close off the tube or make the opening inside smaller - they squeeze. These muscles can contract in a synchronized way to move the food in one direction (usually downstream, but occasionally upstream for short distances); this is called peristalsis. If you looked at the intestine, you would see a ring of contraction that moves along pushing contents ahead of it. At other times, the muscles in adjacent parts of the gastrointestinal tract squeeze more or less independently of each other: this has the effect of mixing the contents but not moving them up or down. Both kinds of contraction patterns are called motility. The gastrointestinal tract is divided into four distinct parts: the esophagus, stomach, small intestine, and large intestine (colon). They are separated from each other by special muscles called sphincters which normally stay tightly closed and which regulate the movement of food and food residues from one part to another. Each part of the gastrointestinal tract has a unique function to perform in digestion, and as a result each part has a distinct type of motility and sensation. When motility or sensations are not appropriate for performing this function, they cause symptoms such as bloating, vomiting, constipation, or diarrhea which are associated with subjective sensations such as pain, bloating, fullness, and urgency to have a bowel movement. -

Distance Between Major and Minor Duodenal Papilla from Pylorus – a Cadaveric Study M
International Journal of Anatomy and Research, Int J Anat Res 2018, Vol 6(2.2):5239-45. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2018.167 DISTANCE BETWEEN MAJOR AND MINOR DUODENAL PAPILLA FROM PYLORUS – A CADAVERIC STUDY M. Pairoja Sultana *1, C.K. Lakshmidevi 2. *1Assistant professor in Anatomy, ACSR Govt. medical college, Nellore, Andhra Pradesh, India. 2 Professor and Head of the department of Anatomy, ACSR Govt. medical college, Nellore, Andhra Pradesh, India. ABSTRACT Introduction: Without the knowledge of the normal pattern of the duct system and its variations, a radiologist can’t interpret an Endoscopic Retrograde Cholangiopancreatography (ERCP) picture. So it becomes important to study the anatomy of pancreatic ducts, their relation to each other, to common bile duct and to duodenum in the available human cadavers. The present paper is about the study of distance between minor and major duodenal papilla from pylorus which was carried out on 96 cadaveric specimens of human duodeno-pancreas. To visualise and to see distance between minor and major duodenal papillae is necessary for the endoscopist who aims to perform the dilation, stenting, or papillotomy of the minor papilla. Materials and Methods: The study was conducted in 96 (64 male and 32 female) cadavers. Major and minor duodenal papillae were visualized through eosin dye installation in both common bile duct and the accessory pancreatic duct. The measurement of distance between the duodenal papillae and to pylorus was done in cm. Results: In the present work, the mean ± SD of the Distance between pylorus to MAP is 8.05 ± 1.71 cm, pylorus to MIP is 6.19 ± 1.49 cm, the major to minor duodenal papilla was on an average 2.02 ± 0.40 cm, these distances were more in males as compared to females. -
Digestive Tract Comparison • the Small Intestine Is a Tube Roughly Twenty Feet Long Deided Into the Duodenum, Jejunum and Ileum
• Small Intestine Human/Dog Digestive system or Simple Monogastric Digestion Digestive Tract Comparison • The small intestine is a tube roughly twenty feet long deided into the duodenum, jejunum and ileum. • The first part of the small intestine is the duodenum, the site of most chemical digestive reactions and is Mouth smoother than the rest of the small intestine • A specialized region of the digestive tract designed to break up large particles of food into • Bile, bicarbonate and pancreatic enzymes are secreted into the duodenum to breakdown nutrients in the smaller, more manageable particles chyme so that they can be readily absorbed. • Saliva is added to moisten food and begin carbohydrate breakdown by amylase in humans. •Bicarbonate from the pancreas neutralizes corrosive stomach acid from 3.5 in the stomach to 8.5 in the • There are four main types of teeth in the human or dog: incisors, canines, premolars and small intestine. molars. •Pancreatic enzymes include lipases, peptidases and amylases. •One reason dog and cat canines are much larger than ours is that they need to be able to rip and •Lipases break down fats. Peptidases break down proteins. Amylases break down carbohydrates. tear through tough raw meat. Humans have evolved to eat easier to chew, cooked meat. • Bile from the liver is stored in the gall bladder and secreted into the duodenum to emulsify fat. • While chewing, food is transformed into what is called a bolus, a food ball, and then forced •The jejunum and ileum are next in the small intestine and are covered in villi, finger-like projections.