Understanding Feline Cardiomyopathy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ischemic Cardiomyopathy: Symptoms, Causes, & Treatment
Ischemic Cardiomyopathy Ischemic cardiomyopathy is a condition that occurs when the heart muscle is weakened due to insufficient blood flow to the heart's muscle. This inhibits the heart's ability to pump blood and can lead to heart failure. What Is Ischemic Cardiomyopathy? Ischemic cardiomyopathy (IC) is a condition that occurs when the heart muscle is weakened. In this condition, the left ventricle, which is the main heart muscle, is usually enlarged and dilated. This condition can be a result of a heart attack or coronary artery disease, a narrowing of the arteries. These narrowed arteries keep blood from reaching portions of your heart. The weakened heart muscle inhibits your heart’s ability to pump blood and can lead to heart failure. Symptoms of IC include shortness of breath, chest pain, and extreme fatigue. If you have IC symptoms, you should seek medical care immediately. Treatment depends on how much damage has been done to your heart. Medications and surgery are often required. You can improve your long-term outlook by making certain lifestyle changes, such as maintaining a healthy diet and avoiding high-risk behaviors, including smoking. Symptoms of Ischemic Cardiomyopathy You can have early-stage heart disease with no symptoms. As the arteries narrow further and blood flow becomes impaired, you may experience a variety of symptoms, including: shortness of breath extreme fatigue dizziness, lightheadedness, or fainting chest pain and pressure (angina) heart palpitations weight gain swelling in the legs and feet (edema) and abdomen difficulty sleeping cough or congestion caused by fluid in the lungs If you have these symptoms, seek emergency medical care or call 9-1-1. -

Myocarditis and Cardiomyopathy
CE: Tripti; HCO/330310; Total nos of Pages: 6; HCO 330310 REVIEW CURRENT OPINION Myocarditis and cardiomyopathy Jonathan Buggey and Chantal A. ElAmm Purpose of review The aim of this study is to summarize the literature describing the pathogenesis, diagnosis and management of cardiomyopathy related to myocarditis. Recent findings Myocarditis has a variety of causes and a heterogeneous clinical presentation with potentially life- threatening complications. About one-third of patients will develop a dilated cardiomyopathy and the pathogenesis is a multiphase, mutlicompartment process that involves immune activation, including innate immune system triggered proinflammatory cytokines and autoantibodies. In recent years, diagnosis has been aided by advancements in cardiac MRI, and in particular T1 and T2 mapping sequences. In certain clinical situations, endomyocardial biopsy (EMB) should be performed, with consideration of left ventricular sampling, for an accurate diagnosis that may aid treatment and prognostication. Summary Although overall myocarditis accounts for a minority of cardiomyopathy and heart failure presentations, the clinical presentation is variable and the pathophysiology of myocardial damage is unique. Cardiac MRI has significantly improved diagnostic abilities, but endomyocardial biopsy remains the gold standard. However, current treatment strategies are still focused on routine heart failure pharmacotherapies and supportive care or cardiac transplantation/mechanical support for those with end-stage heart failure. Keywords cardiac MRI, cardiomyopathy, endomyocardial biopsy, myocarditis INTRODUCTION prevalence seen in children and young adults aged Myocarditis refers to inflammation of the myocar- 20–30 years [1]. dium and may be caused by infectious agents, systemic diseases, drugs and toxins, with viral infec- CAUSE tions remaining the most common cause in the developed countries [1]. -

ICD-10-CM Documentation and Coding Best Practices
ICD-10-CM Documentation and Coding Best Practices Cardiomyopathy Cardiomyopathy refers to diseases of the heart muscle, which can become enlarged, thick or rigid. In rare cases, cardiac muscle tissue can be replaced with scar tissue. As the condition worsens, the heart becomes weaker and less able to pump blood through the body or to maintain a normal electrical rhythm. Causes Risk factors that can increase the possibility of developing cardiomyopathy include: coronary artery disease, a history of heart attack (s), viral infections that cause heart inflammation, long-term hypertension or alcoholism, obesity, and diabetes to name a few. However, in most cases the exact cause is usually unknown (called primary or idiopathic cardiomyopathy). Symptoms Some patients will never have symptoms; others will not develop them until later in the disease. Symptoms can include: • Fatigue • Shortness of b reath or trouble breathing (dyspnea) • Dizziness, lightheadedness or fainting • Swelling in the ankles, feet, legs, abdomen and neck veins Treatment Treatment depends on the type of cardiomyopathy, the severity of symptoms, and the patient’s age and overall health. • Lifestyle changes can help manage condition(s) that may be causing cardiomyopathy. Recommendations include: o Consuming a heart healthy diet, engaging in physical activity, losing excess weight, giving up smoking, avoiding alcohol and illegal drugs, getting enough sleep, and reducing stress • Medicines may be p rescribed to: o Lower blood pressure (ACE inhibitors, angiotensin II receptor blo ckers, beta blockers, calcium channel blockers ) o Slow the heart rate (beta blockers, calcium channel blockers, digoxin) o Prevent arrhythmias (antiarrhythmics) o Remove excess fluid and sodium (diuretics) o Prevent blood clots (anticoagulants) • Alcohol septal ablation • Surgery o Septal myectomy – option for severe cases of obstructive hypertrophic cardiomyopathy Surgically implanted devices o . -

The Pulmonary Manifestations of Left Heart Failure*
The Pulmonary Manifestations of Left Heart Failure* Brian K. Gehlbach, MD; and Eugene Geppert, MD Determining whether a patient’s symptoms are the result of heart or lung disease requires an understanding of the influence of pulmonary venous hypertension on lung function. Herein, we describe the effects of acute and chronic elevations of pulmonary venous pressure on the mechanical and gas-exchanging properties of the lung. The mechanisms responsible for various symptoms of congestive heart failure are described, and the significance of sleep-disordered breathing in patients with heart disease is considered. While the initial clinical evaluation of patients with dyspnea is imprecise, measurement of B-type natriuretic peptide levels may prove useful in this setting. (CHEST 2004; 125:669–682) Key words: Cheyne-Stokes respiration; congestive heart failure; differential diagnosis; dyspnea; pulmonary edema; respiratory function tests; sleep apnea syndromes Abbreviations: CHF ϭ congestive heart failure; CSR-CSA ϭ Cheyne-Stokes respiration with central sleep apnea; CPAP ϭ continuous positive airway pressure; Dlco ϭ diffusing capacity of the lung for carbon monoxide; DM ϭ membrane conductance; FRC ϭ functional residual capacity; OSA ϭ obstructive sleep apnea; TLC ϭ total lung ϭ ˙ ˙ ϭ capacity; VC capillary volume; Ve/Vco2 ventilatory equivalent for carbon dioxide early 5 million Americans have congestive heart For a detailed review of the pathophysiology of N failure (CHF), with 400,000 new cases diag- high-pressure pulmonary edema, the reader is re- nosed each year.1 Unfortunately, despite the consid- ferred to several excellent recent reviews.2–4 erable progress that has been made in understanding the pathophysiology of pulmonary edema, the pul- monary complications of this condition continue to The Pathophysiology of Pulmonary challenge the bedside clinician. -

Percutaneous Mitral Valve Therapies: State of the Art in 2020 LA ACP Annual Meeting
Percutaneous Mitral Valve Therapies: State of the Art in 2020 LA ACP Annual Meeting Steven R Bailey MD MSCAI, FACC, FAHA,FACP Professor and Chair, Department of Medicine Malcolm Feist Chair of Interventional Cardiology LSU Health Shreveport Professor Emeritus, UH Health San Antonio [email protected] SRB March 2020 Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company • Grant/Research Support • None • Consulting Fees/Honoraria • BSCI, Abbot DSMB • Intellectual Property Rights • UTHSCSA • Other Financial Benefit • CCI Editor In Chief SRB March 2020 The 30,000 Ft View Maria SRB March 2020 SRB March 2020 Mitral Stenosis • The most common etiology of MS is rheumatic fever, with a latency of approximately 10 to 20 years after the initial streptococcal infection. Symptoms usually appear in adulthood • Other etiologies are rare but include: congenital MS radiation exposure atrial myxoma mucopolysaccharidoses • MS secondary to calcific annular disease is increasingly seen in elderly patients, and in patients with advanced chronic kidney disease. SRB March 2020 Mitral Stenosis • Mitral stenosis most commonly results from rheumatic heart disease fusion of the valve leaflet cusps at the commissures thickening and shortening of the chordae calcium deposition within the valve leaflets • Characteristic “fish-mouth” or “hockey stick” appearance on the echocardiogram (depending on view) SRB March 2020 Mitral Stenosis: Natural History • The severity of symptoms depends primarily on the degree of stenosis. • Symptoms often go unrecognized by patient and physician until significant shortness of breath, hemoptysis, or atrial fibrillation develops. -

Hypertrophic Cardiomyopathy Guide
Hypertrophic Cardiomyopathy Guide HYPERTROPHIC CARDIOMYOPATHY GUIDE What is hypertrophic cardiomyopathy? Hypertrophic cardiomyopathy (HCM) is a complex type of heart disease that affects the heart muscle. It causes thickening of the heart muscle (especially the ventricles, or lower heart chambers), left ventricular stiffness, mitral valve changes and cellular changes. Thickening of the heart muscle (myocardium) occurs most commonly at the septum. The septum is the muscular wall that separates the left and right side of aortic valve narrowed the heart. Problems occur outflow tract when the septum between outflow tract the heart’s lower chambers, leaky mitral mitral valve or ventricles, is thickened. valve septum The thickened septum may thickened cause a narrowing that can septum block or reduce the blood flow from the left ventricle Normal Heart Hypertrophic to the aorta - a condition Cardiomyopathy called “outflow tract obstruction.” The ventricles must pump harder to overcome the narrowing or blockage. This type of hypertrophic cardiomyopathy may be called hypertrophic obstructive cardiomyopathy (HOCM). HCM also may cause thickening in other parts of the heart muscle, such as the bottom of the heart called the apex, right ventricle, or throughout the entire left ventricle. Stiffness in the left ventricle occurs as a result of cellular changes that occur in the heart muscle when it thickens. The left ventricle is unable to relax normally and fill with blood. Since there is less blood at the end of filling, there is less oxygen-rich blood pumped to the organs and muscles. The stiffness in the left ventricle causes pressure to increase inside the heart and may lead to the symptoms described below. -

An Uncommon Clinical Presentation of Relapsing Dilated Cardiomyopathy with Identification of Sequence Variations in MYNPC3, KCNH2 and Mitochondrial Trna Cysteine M
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by George Washington University: Health Sciences Research Commons (HSRC) Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Pediatrics Faculty Publications Pediatrics 6-2015 An uncommon clinical presentation of relapsing dilated cardiomyopathy with identification of sequence variations in MYNPC3, KCNH2 and mitochondrial tRNA cysteine M. J. Guillen Sacoto Kimberly A. Chapman George Washington University D. Heath M. B. Seprish Dina Zand George Washington University Follow this and additional works at: http://hsrc.himmelfarb.gwu.edu/smhs_peds_facpubs Part of the Pediatrics Commons Recommended Citation Guillen Sacoto, M.J., Chapman, K.A., Heath, D., Seprish, M.B., Zand, D.J. (2015). An uncommon clinical presentation of relapsing dilated cardiomyopathy with identification of sequence variations in MYNPC3, KCNH2 and mitochondrial tRNA cysteine. Molecular Genetics and Metabolism Reports, 3, 47-54. doi:10.1016/j.ymgmr.2015.03.007 This Journal Article is brought to you for free and open access by the Pediatrics at Health Sciences Research Commons. It has been accepted for inclusion in Pediatrics Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. Molecular Genetics and Metabolism Reports 3 (2015) 47–54 Contents lists available at ScienceDirect Molecular Genetics and Metabolism Reports journal homepage: http://www.journals.elsevier.com/molecular-genetics-and- metabolism-reports/ Case Report An uncommon clinical presentation of relapsing dilated cardiomyopathy with identification of sequence variations in MYNPC3, KCNH2 and mitochondrial tRNA cysteine Maria J. Guillen Sacoto a,1, Kimberly A. -

Currentstateofknowledgeonaetiol
European Heart Journal (2013) 34, 2636–2648 ESC REPORT doi:10.1093/eurheartj/eht210 Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases Downloaded from Alida L. P. Caforio1†*, Sabine Pankuweit2†, Eloisa Arbustini3, Cristina Basso4, Juan Gimeno-Blanes5,StephanB.Felix6,MichaelFu7,TiinaHelio¨ 8, Stephane Heymans9, http://eurheartj.oxfordjournals.org/ Roland Jahns10,KarinKlingel11, Ales Linhart12, Bernhard Maisch2, William McKenna13, Jens Mogensen14, Yigal M. Pinto15,ArsenRistic16, Heinz-Peter Schultheiss17, Hubert Seggewiss18, Luigi Tavazzi19,GaetanoThiene4,AliYilmaz20, Philippe Charron21,andPerryM.Elliott13 1Division of Cardiology, Department of Cardiological Thoracic and Vascular Sciences, University of Padua, Padova, Italy; 2Universita¨tsklinikum Gießen und Marburg GmbH, Standort Marburg, Klinik fu¨r Kardiologie, Marburg, Germany; 3Academic Hospital IRCCS Foundation Policlinico, San Matteo, Pavia, Italy; 4Cardiovascular Pathology, Department of Cardiological Thoracic and Vascular Sciences, University of Padua, Padova, Italy; 5Servicio de Cardiologia, Hospital U. Virgen de Arrixaca Ctra. Murcia-Cartagena s/n, El Palmar, Spain; 6Medizinische Klinik B, University of Greifswald, Greifswald, Germany; 7Department of Medicine, Heart Failure Unit, Sahlgrenska Hospital, University of Go¨teborg, Go¨teborg, Sweden; 8Division of Cardiology, Helsinki University Central Hospital, Heart & Lung Centre, -

Atrial Fibrillation in Hypertrophic Cardiomyopathy: Prevalence, Clinical Impact, and Management
Heart Failure Reviews (2019) 24:189–197 https://doi.org/10.1007/s10741-018-9752-6 Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical impact, and management Lohit Garg 1 & Manasvi Gupta2 & Syed Rafay Ali Sabzwari1 & Sahil Agrawal3 & Manyoo Agarwal4 & Talha Nazir1 & Jeffrey Gordon1 & Babak Bozorgnia1 & Matthew W. Martinez1 Published online: 19 November 2018 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract Hypertrophic cardiomyopathy (HCM) is the most common hereditary cardiomyopathy characterized by left ventricular hyper- trophy and spectrum of clinical manifestation. Atrial fibrillation (AF) is a common sustained arrhythmia in HCM patients and is primarily related to left atrial dilatation and remodeling. There are several clinical, electrocardiographic (ECG), and echocardio- graphic (ECHO) features that have been associated with development of AF in HCM patients; strongest predictors are left atrial size, age, and heart failure class. AF can lead to progressive functional decline, worsening heart failure and increased risk for systemic thromboembolism. The management of AF in HCM patient focuses on symptom alleviation (managed with rate and/or rhythm control methods) and prevention of complications such as thromboembolism (prevented with anticoagulation). Finally, recent evidence suggests that early rhythm control strategy may result in more favorable short- and long-term outcomes. Keywords Atrial fibrillation . Hypertrophic cardiomyopathy . Treatment . Antiarrhythmic agents Introduction amyloidosis) [3–5]. The clinical presentation of HCM is het- erogeneous and includes an asymptomatic state, heart failure Hypertrophic cardiomyopathy (HCM) is the most common syndrome due to diastolic dysfunction or left ventricular out- inherited cardiomyopathy due to mutation in one of the sev- flow (LVOT) obstruction, arrhythmias (atrial fibrillation and eral sarcomere genes and transmitted in autosomal dominant embolism), and sudden cardiac death [1, 6]. -

Coronary Microvascular Dysfunction
Journal of Clinical Medicine Review Coronary Microvascular Dysfunction Federico Vancheri 1,*, Giovanni Longo 2, Sergio Vancheri 3 and Michael Henein 4,5,6 1 Department of Internal Medicine, S.Elia Hospital, 93100 Caltanissetta, Italy 2 Cardiovascular and Interventional Department, S.Elia Hospital, 93100 Caltanissetta, Italy; [email protected] 3 Radiology Department, I.R.C.C.S. Policlinico San Matteo, 27100 Pavia, Italy; [email protected] 4 Institute of Public Health and Clinical Medicine, Umea University, SE-90187 Umea, Sweden; [email protected] 5 Department of Fluid Mechanics, Brunel University, Middlesex, London UB8 3PH, UK 6 Molecular and Nuclear Research Institute, St George’s University, London SW17 0RE, UK * Correspondence: [email protected] Received: 10 August 2020; Accepted: 2 September 2020; Published: 6 September 2020 Abstract: Many patients with chest pain undergoing coronary angiography do not show significant obstructive coronary lesions. A substantial proportion of these patients have abnormalities in the function and structure of coronary microcirculation due to endothelial and smooth muscle cell dysfunction. The coronary microcirculation has a fundamental role in the regulation of coronary blood flow in response to cardiac oxygen requirements. Impairment of this mechanism, defined as coronary microvascular dysfunction (CMD), carries an increased risk of adverse cardiovascular clinical outcomes. Coronary endothelial dysfunction accounts for approximately two-thirds of clinical conditions presenting with symptoms and signs of myocardial ischemia without obstructive coronary disease, termed “ischemia with non-obstructive coronary artery disease” (INOCA) and for a small proportion of “myocardial infarction with non-obstructive coronary artery disease” (MINOCA). More frequently, the clinical presentation of INOCA is microvascular angina due to CMD, while some patients present vasospastic angina due to epicardial spasm, and mixed epicardial and microvascular forms. -
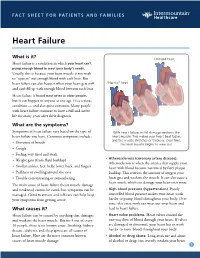
Heart Failure
FACT SHEET FOR PATIENTS AND FAMILIES Heart Failure What is it? Enlarged heart Heart failure is a condition in which your heart can’t pump enough blood to meet your body’s needs. Usually, this is because your heart muscle is too weak to “squeeze” out enough blood with each beat. But heart failure can also happen when your heart gets stiff “Normal” heart and can’t fill up with enough blood between each beat. Heart failure is found most often in older people, but it can happen to anyone at any age. It’s a serious condition — and also quite common. Many people with heart failure continue to have a full and active life for many years after their diagnosis. What are the symptoms? Symptoms of heart failure vary based on the type of With heart failure, initial damage weakens the heart failure you have. Common symptoms include: heart muscle. This makes your heart beat faster, and the muscle stretches or thickens. Over time, • Shortness of breath the heart muscle begins to wear out. • Cough • Feeling very tired and weak • Atherosclerosis (coronary artery disease). • Weight gain (from fluid buildup) Atherosclerosis is when the arteries that supply your • Swollen ankles, feet, belly, lower back, and fingers heart with blood become narrowed by fatty plaque • Puffiness or swelling around the eyes buildup. This restricts the amount of oxygen your • Trouble concentrating or remembering heart gets and weakens the muscle. It can also cause a heart attack, which can damage your heart even more. The main cause of heart failure (heart muscle damage and weakness) cannot be cured, but symptoms can be • High blood pressure (hypertension). -

Hypertrophic Cardiomyopathy
HYPERTROPHIC CARDIOMYOPATHY Most often diagnosed during infancy or adolescence, hypertrophic cardiomyopathy (HCM) is the second most common form of heart muscle disease, is usually genetically transmitted, and comprises about 35–40% of cardiomyopathies in children. A diagram and echocardiogram comparing a normal heart and a heart with HCM are shown in figures 2a and 2b. Figure 2a- A normal Figure 2b- Multiple heart is shown on echocardiographic the left compared views of a normal to a heart with a heart on the left hypertrophic and a heart with cardiomyopathy on hypertrophic the right. Note the cardiomyopathy increased thickness on the right. Note of the walls of the the increased left ventricle. thickness of the walls of the left ventricle (LV). HCM affects up to 500,000 people in the United States. with children under age 12 accounting for less than 10% of all cases. According to the Pediatric Cardiomyopathy Registry, HCM occurs at a rate of five per 1 million children. “Hypertrophic” refers to an abnormal growth of muscle fibers in the heart. In HCM, the thick heart muscle is stiff, making it difficult for the heart to relax and for blood to fill the heart chambers. While the heart squeezes normally, the limited filling prevents the heart from pumping enough blood, especially during exercise. Although HCM can involve both lower chambers, it usually affects the main pumping chamber (left ventricle) with thickening of the septum (wall separating the pumping chambers), posterior wall or both. With hypertrophic obstructive cardiomyopathy (HOCM), the muscle thickening restricts the flow of blood out of the heart.