Origins of Flavour in Whiskies and a Revised Flavour Wheel: a Review, Pp.287-313 Volume 107, No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lavender- and Lavandin-Distilled Straws: an Untapped Feedstock With
Lesage‑Meessen et al. Biotechnol Biofuels (2018) 11:217 https://doi.org/10.1186/s13068-018-1218-5 Biotechnology for Biofuels RESEARCH Open Access Lavender‑ and lavandin‑distilled straws: an untapped feedstock with great potential for the production of high‑added value compounds and fungal enzymes Laurence Lesage‑Meessen1, Marine Bou1, Christian Ginies2, Didier Chevret3, David Navarro1, Elodie Drula1,4, Estelle Bonnin5, José C. del Río6, Elise Odinot1, Alexandra Bisotto1, Jean‑Guy Berrin1, Jean‑Claude Sigoillot1, Craig B. Faulds1 and Anne Lomascolo1* Abstract Background: Lavender (Lavandula angustifolia) and lavandin (a sterile hybrid of L. angustifolia L. latifolia) essential oils are among those most commonly used in the world for various industrial purposes, including× perfumes, phar‑ maceuticals and cosmetics. The solid residues from aromatic plant distillation such as lavender- and lavandin-distilled straws are generally considered as wastes, and consequently either left in the felds or burnt. However, lavender- and lavandin-distilled straws are a potentially renewable plant biomass as they are cheap, non-food materials that can be used as raw feedstocks for green chemistry industry. The objective of this work was to assess diferent pathways of valorization of these straws as bio-based platform chemicals and fungal enzymes of interest in biorefnery. Results: Sugar and lignin composition analyses and saccharifcation potential of the straw fractions revealed that these industrial by-products could be suitable for second-generation bioethanol prospective. The solvent extrac‑ tion processes, developed specifcally for these straws, released terpene derivatives (e.g. τ-cadinol, β-caryophyllene), lactones (e.g. coumarin, herniarin) and phenolic compounds of industrial interest, including rosmarinic acid which contributed to the high antioxidant activity of the straw extracts. -

The Analysis of Grapevine Response to Smoke Exposure
The Analysis of Grapevine Response to Smoke Exposure A thesis presented in fulfilment of the requirements for the degree of Doctor of Philosophy Lieke van der Hulst BSc, MSc The University of Adelaide School of Agriculture, Food and Wine Thesis submitted for examination: November 2017 Thesis accepted and final submission: January 2018 Table of contents Abstract i Declaration iii Publications iv Symposia v Acknowledgements vii Chapter 1 Literature review and introduction • Literature review and introduction 1 • The occurrence of bushfires and prescribed 2 • Economic impact of bushfires 5 • Smoke derived volatile compounds 6 • Volatile compounds in wine 8 • Glycosylation of volatile phenols in grapes 9 • Previous smoke taint research 11 • Glycosyltransferases 14 • Research aims 18 Chapter 2 Detection and mitigation of smoke taint in the vineyard • Authorship statements 20 • Introduction 22 • Paper: Accumulation of volatile phenol glycoconjugates in grapes, 24 following the application of kaolin and/or smoke to grapevines (Vitis vinifera cv Sauvignon Blanc, Chardonnay and Merlot) • Further investigation into methods for the detection and mitigation of 54 smoke taint in the vineyard Material and Methods 55 Results and discussion part A 57 Results and discussion part B 61 Conclusion 68 Chapter 3 Expression of glycosyltransferases in grapevines following smoke exposure • Authorship statements 71 • Introduction 73 • Paper: Expression profiles of glycosyltransferases in 74 Vitis vinifera following smoke exposure Chapter 4 The effect of smoke exposure to apple • Authorship statements 122 • Introduction 124 • Paper: The effect of smoke exposure to apple (Malus domestica 125 Borkh cv ‘Sundowner’) Chapter 5 Conclusions and future directions • Conclusions 139 • Future directions 142 Appendix • Paper: Impact of bottle aging on smoke-tainted wines from 145 different grape cultivars References 152 Abstract Smoke taint is a fault found in wines made from grapes exposed to bushfire smoke. -

The Evolution of the UK Wine Market: from Niche to Mass-Market Appeal
beverages Article The Evolution of the UK Wine Market: From Niche to Mass-Market Appeal Julie Bower Independent Scholar, Worcester WR1 3DG, UK; [email protected] Received: 4 October 2018; Accepted: 8 November 2018; Published: 12 November 2018 Abstract: This article is an historic narrative account of the emergence of the mass-market wine category in the UK in the post-World War II era. The role of the former vertically-integrated brewing industry in the early stages of development is described from the perspective of both their distributional effects and their new product development initiatives. Significant in the narrative is the story of Babycham, the UK’s answer to Champagne that was targeted to the new consumers of the 1950s; women. Then a specially-developed French wine, Le Piat D’Or, with its catchy advertising campaign, took the baton. These early brands were instrumental in extending the wine category, as beer continued its precipitous decline. That the UK is now one of the largest wine markets globally owes much to the success of these early brands and those that arrived later in the 1990s, with Australia displacing France as the source for mass-market appeal. Keywords: UK wine consumption; UK brewing industry; resource partitioning theory; targeted marketing 1. Introduction The evolution of wine consumption in the UK is described by important socio-economic trends in consumer behavior that emerged in the 1950s. This coincided with a growing awareness within the alcoholic beverages industry that there was the need for new product development to satisfy the increasingly sophisticated and aspirational consumer. -

Lactic Acid Bacteria – the Uninvited but Generally Welcome Participants In
Lactic acid bacteria – the uninvited (a) but generally welcome participants in malt whisky fermentation Fergus G. Priest Coffey still. Like malt diversity resulting in Lactobacillus fermentum and LEFT: (b) whisky, it must be matured Lactobacillus paracasei as commonly dominant species by Fig. 1. Fluorescence photomicrographs of whisky for a minimum of 3 years. about 40 hours. During the final stages when the yeast is fermentation samples; green cells Grain whisky is the base of dying, a homofermentative bacterium related to Lacto- are viable, red cells are dead. (a) the standard blends such as bacillus acidophilus often proliferates and produces large Wort on entering the washback, (b) Famous Grouse, Teachers, amounts of lactic acid. after fermentation for 55 hours and and Johnnie Walker which (c) after fermentation for 95 hours. (a) AND (b) ARE REPRODUCED will contain between 15 Effects of lactobacilli on the flavour of malt WITH PERMISSION FROM VAN BEEK & and 30 % malt whisky. whisky PRIEST (2002, APPL ENVIRON Both products use a The bacteria can affect the flavour of the spirit in two MICROBIOL 68, 297–305); (c) COURTESY F. G. PRIEST similar process, but here ways. First, they will reduce the pH of the fermentation we will focus on malt (c) through the production of acetic and lactic acids. This whisky. Malted barley is will lead to a general increase in esters following milled, infused in water at distillation, a positive feature that has traditionally been about 64 °C for some 30 associated with the late lactic fermentation. This general minutes to 1 hour and the effect is apparent in the data presented in Fig. -

CPY Document
3. eHEMieAL eOMPOSITION OF ALeOHOLie BEVERAGES, ADDITIVES AND eONTAMINANTS 3.1 General aspects Ethanol and water are the main components of most alcoholIc beverages, although in some very sweet liqueurs the sugar content can be higher than the ethanol content. Ethanol (CAS Reg. No. 64-17-5) is present in alcoholic beverages as a consequence of the fermentation of carbohydrates with yeast. It can also be manufactured from ethylene obtained from cracked petroleum hydrocarbons. The a1coholic beverage industry has generally agreed not to use synthetic ethanol manufactured from ethylene for the production of alcoholic beverages, due to the presence of impurities. ln order to determine whether synthetic ethanol has been used to fortify products, the low 14C content of synthetic ethanol, as compared to fermentation ethanol produced from carbohydrates, can be used as a marker in control analyses (McWeeny & Bates, 1980). Some physical and chemical characteristics of anhydrous ethanol are as follows (Windholz, 1983): Description: Clear, colourless liquid Boilng-point: 78.5°C M elting-point: -114.1 °C Density: d¡O 0.789 It is widely used in the laboratory and in industry as a solvent for resins, fats and oils. It also finds use in the manufacture of denatured a1cohol, in pharmaceuticals and cosmetics (lotions, perfumes), as a chemica1 intermediate and as a fuel, either alone or in mixtures with gasolIne. Beer, wine and spirits also contain volatile and nonvolatile flavour compounds. Although the term 'volatile compound' is rather diffuse, most of the compounds that occur in alcoholIc beverages can be grouped according to whether they are distiled with a1cohol and steam, or not. -

Alcohol Units a Brief Guide
Alcohol Units A brief guide 1 2 Alcohol Units – A brief guide Units of alcohol explained As typical glass sizes have grown and For example, most whisky has an ABV of 40%. popular drinks have increased in A 1 litre (1,000ml) bottle of this whisky therefore strength over the years, the old rule contains 400ml of pure alcohol. This is 40 units (as 10ml of pure alcohol = one unit). So, in of thumb that a glass of wine was 100ml of the whisky, there would be 4 units. about 1 unit has become out of date. And hence, a 25ml single measure of whisky Nowadays, a large glass of wine might would contain 1 unit. well contain 3 units or more – about the The maths is straightforward. To calculate units, same amount as a treble vodka. take the quantity in millilitres, multiply it by the ABV (expressed as a percentage) and divide So how do you know how much is in by 1,000. your drink? In the example of a glass of whisky (above) the A UK unit is 10 millilitres (8 grams) of pure calculation would be: alcohol. It’s actually the amount of alcohol that 25ml x 40% = 1 unit. an average healthy adult body can break down 1,000 in about an hour. So, if you drink 10ml of pure alcohol, 60 minutes later there should be virtually Or, for a 250ml glass of wine with ABV 12%, none left in your bloodstream. You could still be the number of units is: suffering some of the effects the alcohol has had 250ml x 12% = 3 units. -

Know Your Liquor VODKA WHISKY
Know Your Liquor VODKA Good to know: Vodka is least likely to give you a hangover Vodka is made by fermenting grains or crops such as potatoes with yeast. It's then purified and repeatedly filtered, often through charcoal, strange as it sounds, until it's as clear as possible. CALORIES: Because vodka contains no carbohydrates or sugars, it contains only calories from ethanol (around 7 calories per gram), making it the least-fattening alcoholic beverage. So a 35ml shot of vodka would contain about 72 calories. PROS: Vodka is the 'cleanest' alcoholic beverage because it contains hardly any 'congeners' - impurities normally formed during fermentation.These play a big part in how bad your hangover is. Despite its high alcohol content - around 40 per cent - vodka is the least likely alcoholic drink to leave you with a hangover, said a study by the British Medical Association CONS: Vodka is often a factor in binge drinking deaths because it is relatively tasteless when mixed with fruit juices or other drinks. HANGOVER SEVERITY: 3/10 WHISKY Good to know: Whisky or Scotch is distilled from fermented grains, such as barley or wheat, then aged in wooded casks. Whisky 'madness': It triggers erratic and unpredictable behaviour because most people drink whisky neat CALORIES: About 80 calories per 35ml shot. PROS: Single malt whiskies have been found to contain high levels of ellagic acid, according to Dr Jim Swan of the Royal Society of Chemists. This powerful acid inhibits the growth of tumours caused by certain carcinogens and kills cancer cells without damaging healthy cells. -

An Investigation of the Voltammetric Behaviour of Antioxidants in Flavonoids
AN INVESTIGATION OF THE VOLTAMMETRIC BEHAVIOUR OF ANTIOXIDANTS IN FLAVONOIDS By Lee-Ann Ramsarup Student Number: 20427580 Submitted in fulfilment of the requirements for the degree of Master of Applied Science in Chemistry in the Faculty of Applied Sciences at the Durban University of Technology April 2020 DECLARATION I, Lee-Ann Ramsarup, hereby declare that the work presented in this thesis entitle “An Investigation of the Voltammetric Behaviour of Antioxidants in Flavonoids” has not been submitted to any other University or Institution for any degree or examination. This is my own work and where the work of others was used, it was to the best of my knowledge accurately reported and duly acknowledged. Signature of Lee-Ann Ramsarup (Student) Date Signature of Prof Krishna Bisetty (Supervisor) Date Signature of Dr Suvardhan Kanchi (Co- Supervisor) Date i DEDICATION This work is dedicated to my late uncle, Gayson Samuel. I am thankful for his presence and support throughout my life. His memory lives on forever. ii ACKNOWLEDGMENTS All that I have and all that I am is because of my Lord and Saviour Jesus Christ. All honour and glory to Him for giving me the strength and faith to see this project to completion. I wish to express my sincere appreciation to my Supervisor, Prof Krishna Bisetty for his assistance and constant motivation throughout my research. I extend my heartfelt appreciation to my Co-Supervisor, Dr Suvardhan Kanchi for his time, support and direction during this journey. Special thanks to Dr Myalo I Sabela (Lecturer) and Nokukhanya Xhakaza (Technician) for assisting me with their time and experience during the experimental phase of the research work. -

The Effects of Combining Guaiacol and Syringol on Their Pyrolysis
Holzforschung, Vol. 66, pp. 323–330, 2012 • Copyright © by Walter de Gruyter • Berlin • Boston. DOI 10.1515/HF.2011.165 The effects of combining guaiacol and syringol on their pyrolysis Mohd Asmadi, Haruo Kawamoto * and Shiro Saka methods, lignin pyrolysis has been studied based on iso- Graduate School of Energy Science , Kyoto University, lated lignins (Martin et al. 1979 ; Obst 1983 ; Evans et al. Kyoto , Japan 1986 ; Saiz -Jimenez and De Leeuw 1986 ; Faix et al. 1987 ; Genuit et al. 1987 ; Meier and Faix 1992 ; Greenwood et al. * Corresponding author. 2002 ; Hosoya et al. 2007, 2008a,b ; Nakamura et al. 2008 ) Graduate School of Energy Science, Kyoto University, and model compounds (Domburg et al. 1974 ; Bre ž n ý et al. Yoshida-honmachi, Sakyo-ku, Kyoto 606-8501, Japan 1983 ; Saiz -Jimenez and De Leeuw 1986 ; Kawamoto et al. Phone/Fax: + 81-75-753-4737 E-mail: [email protected] 2006, 2007, 2008a,b ; Kawamoto and Saka 2007 ; Nakamura et al. 2007, 2008 ; Watanabe et al. 2009 ). Hardwood lignins are known to include both the syringyl (3,5-dimethoxy-4- Abstract hydroxyphenyl)-type (shortly S) and the guaiacyl (4-hydroxy- 3-methoxyphenyl)-type aromatic rings (shortly G), whereas Pyrolysis of guaiacol/syringol mixtures was studied in an softwood lignins contain mainly the G-type units. These dif- ° ferences infl uence the pyrolytic reactivity of hardwoods and ampoule reactor (N 2 /600 C/40 – 600 s) to understand the reactivities of the aromatic nuclei in hardwood lignins. By softwoods (Di Blasi et al. 2001a,b ; Gr ø nli et al. -
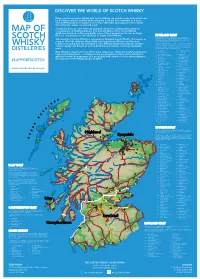
2019 Scotch Whisky
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character. -

Gewinner 2009
LAND / KAT. NAME / CATEGORY AWARD BRAUEREI / BREWERY ORT / LOCATION COUNTRY BIER / BEER WEBSITE Gold Hofbräuhaus Traunstein Josef Sailer KG Traunstein Germany Fürstentrunk www.hb-ts.de Festival Beer / Festbier Silber / Silver Brauhaus Faust OHG Miltenberg Germany Faust Festbier www.faust.de Bronze Brauerei Wiethaler Lauf-Neunhof Germany Wiethaler Goldstoff Hell www.brauerei-wiethaler.de Gold Camba Bavaria GmbH Truchtlaching Germany Trucht´linger Doppelbock www.cambabavaria.de German Style Stichting Noordhollandse Alternatieve Dark Bock / Dunkler Bock Silber / Silver Bierbrouwers Purmerend Netherlands YSBOK www.snab.nl Bronze Schlossbrauerei Autenried GmbH Ichenhausen Germany Leonhardi Bock www.autenrieder.de Gold Bürgerliches Brauhaus Saalfeld GmbH Saalfeld Germany Saalfelder Bock www.brauhaus-saalfeld.de German Style Pale and Amber Bock / Heller und Bernsteinfarbener Silber / Silver Brauerei-Gasthof Kundmüller KG Viereth-Trunstadt Germany Weiherer Bock www.kundmueller.de Bock Lurago Marinone Bronze Nuovo Birrificio Italiano s.r.l. (Como) Italy Bibock www.birrificio.it Private Landbrauerei Schönram A. Gold Oberlindober jun. Petting/Schönram Germany Schönramer Pils www.brauerei-schoenram.de German Style Privatbrauerei M. C. Wieninger GmbH & Pilsner Silber / Silver Co. KG Teisendorf Teisendorf Germany Wieninger Ruperti Pils www.wieninger.de Bronze Trumer Privatbrauerei Josef Sigl Obertrum am See Austria Trumer Pils www.trumer.at Gold Cervejaria Sudbrack Ltda. Blumenau-SC Brasil Eisenbahn Dunkel www.eisenbahn.com.br German Style Scheibenberg/ Schwarzbier Silber / Silver Fiedler-Bräu Erzgebirgsbier Oberscheibe Germany Magisterbräu Schwarzbier www.brauerei-fiedler.de Bronze FX Matt Brewing Company Utica, NY USA Saranac Black Forest www.saranac.com LAND / KAT. NAME / CATEGORY AWARD BRAUEREI / BREWERY ORT / LOCATION COUNTRY BIER / BEER WEBSITE Gold Brauerei Goss Deuerling Germany Goss-Märzen Milwaukee, Bavarian Style Silber / Silver Lakefront Brewing, Inc. -

“Off” Flavors in Beer Their Causes & How to Avoid Them a Moremanual ™ Morebeer.Com 1–800–600–0033
“Off” Flavors In Beer Their Causes & How To Avoid Them A MoreManual ™ MoreBeer.com 1–800–600–0033 Acetaldehyde puckering sensation, may feel powdery or metallic in the mouth, like sucking on a grape skin or a tea bag • Tastes/Smells Like: Green apples, rotten-apples, freshly cut pumpkin. • Possible Causes: Astringency can be caused by many different factors. Polyphenols or tannins are the • Possible Causes: Acetaldehyde is a naturally occurring number one cause of such flavors. Tannins are found chemical produced by yeast during fermentation. It is in the skins or husks of the grain as well as in the skin usually converted into Ethanol alcohol, although this of fruit. Steeping grain for too long or grain that has process may take longer in beers with high alcohol been excessively milled or crushed can release tan- content or when not enough yeast is pitched. Some nins. When mashing, if the pH exceeds 5.2–5.6, as- bacteria can cause green apple flavors as well. tringent flavors can be produced. Over-hopping can • How to Avoid: Let the beer age and condition over also lend a hand in creating astringent qualities. a couple months time. This will give the yeast time • How to Avoid: Avoid grain that has been “over-milled”. to convert the Acetaldehyde into Ethanol. Always use Grain should be cracked open but not crushed or high quality yeast and make sure you are pitching the shredded. When sparging, pay close attention to correct amount for the gravity of the wort or make a the temperature and the amount of the water used.