Functional Antagonism of Voltage-Gated K+ Channel Α-Subunits In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meninges Ventricles And
Meninges ,ventricles & CSF Dr.Sanaa Al-Shaarawy Dr. Essam Eldin Salama OBJECTIVES • By the end of the lecture the student should be able to: • Describe the cerebral meninges & list the main dural folds. • Describe the spinal meninges & locate the level of the termination of each of them. • Describe the importance of the subarachnoid space. • List the Ventricular system of the CNS and locate the site of each of them. • Describe the formation, circulation, drainage, and functions of the CSF. • Know some clinical point about the CSF MENINGES • The brain and spinal cord are invested by three concentric membranes ; • The outermost layer is the dura matter. • The middle layer is the arachnoid matter. • The innermost layer is the pia matter. DURA MATER ▪The cranial dura is a two layered tough, fibrous thick membrane that surrounds the brain. ▪It is formed of two layers; periosteal and meningeal. ▪The periosteal layer is attached to the skull. ▪The meningeal layer is folded forming the dural folds : falx cerebri, and tentorium cerebelli. ▪Sensory innervation of the dura is mostly from : meningeal branches of the trigeminal and vagus nerves & C1 to C3(upper cervical Ns.). DURA MATER Folds Two large reflection of dura extend into the cranial cavity : 1.The falx cerebri, In the midline, ▪It is a vertical sickle-shaped sheet of dura, extends from the cranial roof into the great longitudinal fissure between the two cerebral hemispheres. ▪It has an attached border adherent to the skull. ▪And a free border lies above the corpus callosum. DURA MATER Folds 2. A horizontal shelf of dura, The tentorium cerebelli, ▪ It lies between the posterior part of the cerebral hemispheres and the cerebellum. -

A Recommended Workflow Methodology in the Creation of An
Manson et al. BMC Medical Imaging (2015) 15:44 DOI 10.1186/s12880-015-0088-6 TECHNICAL ADVANCE Open Access A recommended workflow methodology in the creation of an educational and training application incorporating a digital reconstruction of the cerebral ventricular system and cerebrospinal fluid circulation to aid anatomical understanding Amy Manson1,2, Matthieu Poyade2 and Paul Rea1* Abstract Background: The use of computer-aided learning in education can be advantageous, especially when interactive three-dimensional (3D) models are used to aid learning of complex 3D structures. The anatomy of the ventricular system of the brain is difficult to fully understand as it is seldom seen in 3D, as is the flow of cerebrospinal fluid (CSF). This article outlines a workflow for the creation of an interactive training tool for the cerebral ventricular system, an educationally challenging area of anatomy. This outline is based on the use of widely available computer software packages. Methods: Using MR images of the cerebral ventricular system and several widely available commercial and free software packages, the techniques of 3D modelling, texturing, sculpting, image editing and animations were combined to create a workflow in the creation of an interactive educational and training tool. This was focussed on cerebral ventricular system anatomy, and the flow of cerebrospinal fluid. Results: We have successfully created a robust methodology by using key software packages in the creation of an interactive education and training tool. This has resulted in an application being developed which details the anatomy of the ventricular system, and flow of cerebrospinal fluid using an anatomically accurate 3D model. -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -

Estimation of Ventricles Size of Human Brain by Magnetic Resonance
Original Research Article Original Research Article Journal of Gandaki Medical College Nepal Estimation of ventricles size of human brain by Magnetic Resonance Imaging in Nepalese Population: A retrospective study Sushma Singh1* iD , Bhoj Raj Sharma2, Urusha Prajapati3, Pujan Sharma4, Manoj Bhatta3, Nawaraj Poudel2 1Lecturer/B.Sc MIT Programe Coordinator, Department of Radiology Gandaki Medical College 2Lecturer, Department of Radiology Gandaki Medical College 3Radiological Technologist 4Associate Professor, Department of Radiology Gandaki Medical College ABSTRACT Background and Objective: data Magnetic resonance imaging (MRI) provides image acquisition of three-dimensional and measurement in any chosen imaging plane. Objective of this study is to assess the size of ventricles of the dimensions among different age and gender. Materials and methods: This is a cross-sectional retrospective study done brain of normal Nepalese people and establish the range of size of the ventricular system and compute the ventricular at Gandaki Medical College, Pokhara. A total of 106 MRI scan data of healthy individuals were collected over a period of seven months between March to September 2019. Patients ranged between eight and eighty years of age with 58 males and 48 females. Measurements of the mean of bifrontal diameter (BFD), bihemispheric diameter (BHD), third ventricle Result: transverse dimension (TVTD), fourth ventricle antero-posterior dimension (FVAP), fourth ventricle width (FVW), and frontal horn ratio (FHR) were done. The mean of BFD, BHD, TVTD, FVAP, FVW, and FHR were found to be 3.05 ± 0.10 cm, 10.11 ± 0.40 cm, 0.43 ± 0.11 cm, 0.90 ± 0.11 cm, 1.22 ± 0.12 cm, and 0.30 ± 0.01 cm, respectively. -

Morphometric Analysis of Ventricular System of Human Brain - a Study by Dissection Method
Jemds.com Original Research Article Morphometric Analysis of Ventricular System of Human Brain - A Study by Dissection Method Prabahita Baruah1, Purujit Choudhury2, Pradipta Ray Choudhury3 1Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. 2Department of Surgery, Gauhati Medical College and Hospital, Guwahati, Assam, India. 3Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. ABSTRACT BACKGROUND It is often a challenge to determine if the brain ventricles are within normal limits Corresponding Author: or swollen with the age of the patient. With a standardized and comparable system, Dr. Purujit Choudhury, it is therefore necessary to define normal ventricular size ranges. Cadaveric Associate Professor, dissection is always considered the gold standard of anatomical education. Present Department of Surgery, Gauhati Medical College and Hospital, work is undertaken to study morphometric analysis of lateral, third & fourth Guwahati, Assam, India. ventricles by dissection method. Morphometric assessment of the ventricular E-mail: [email protected] system is helpful in the diagnosis as well as classification of hydrocephalus and in the evaluation and monitoring of ventricular system enlargement during DOI: 10.14260/jemds/2020/121 ventricular shunt therapy. Financial or Other Competing Interests: METHODS None. Different parameters of all parts of lateral ventricle, third and fourth ventricle were How to Cite This Article: measured with digital vernier caliper in cadaveric brain specimens. The brain Baruah P, Choudhury P, Choudhury PR. specimens were obtained from dead bodies subjected to post-mortem Morphometric analysis of ventricular examinations in the Department of Forensic Medicine and from the dead bodies system of human brain- a study by voluntarily donated to the Department of Anatomy, Silchar Medical College, Silchar. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
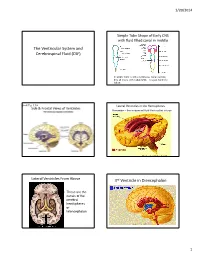
The Ventricular System and Cerebrospinal Fluid (CSF) 3Rd Ventricle in Diencephalon
1/20/2014 Simple Tube Shape of Early CNS with fluid filled canal in middle The Ventricular System and Cerebrospinal Fluid (CSF) In adults there is still a continuous canal running thru all levels of the adult CNS – it is just harder to follow. Book Fig. 1.19 Lateral Ventricles in the Hemispheres Side & Frontal Views of Ventricles Remember – these represent fluid filled cavities in brain “Wishbone” shape means there is ventricle within each of the lobes. Lateral Ventricles From Above 3rd Ventricle in Diencephalon • These are the canals of the cerebral hemispheres or telencephalon 1 1/20/2014 Interventricular foramen links lateral ventricles to 3rd You can see the 2 dark lateral ventricles as well as the vertical midline 3 rd ventricle Sagittal Section Thru 3 rd Vent. (#2 in figure) • Connects to skinny cerebral aqueduct, the canal of the midbrain (just under the #10) Choroid plexus located in ventricles continuously 4th Ventricle in the Hindbrain produces CSF, replacing the ~125-150 ml several times/day Normal pressure 3 holes in the roof of CSF: of the 4 th venticle 100-150 mmH2O allow most CSF to lying down leave ventricles 200-300 mmH2O and enter the sitting up subarachnoid space around brain 2 1/20/2014 Circulation of CSF in • Why is CSF pressure measured in mm H2O? • Larger pressure differences (like BP) are ventricles measured by the movement of a column of always heavier liquid (Mercury or Hg) moves • Smaller pressure differences are measured by the movement of a column of lighter liquid (H2O) posteriorly, – CSF moves that column ~150 mm but would toward only move Hg about 2 mm. -

Hydrocephalus Really Communicating? Prospective Study on the Value of 3D-Constructive Interference in Steady State ORIGINAL RESEARCH Sequence at 3T
Published July 30, 2009 as 10.3174/ajnr.A1726 Is All “Communicating” Hydrocephalus Really Communicating? Prospective Study on the Value of 3D-Constructive Interference in Steady State ORIGINAL RESEARCH Sequence at 3T A. Dinçer BACKGROUND AND PURPOSE: 3D-constructive interference in steady state (3D-CISS) sequence has S. Kohan been used to assess the CSF pathways. The aim of this study was to investigate the additive value of 3D-CISS compared with conventional sequences in the diagnosis of obstructive membranes in M.M. O¨ zek hydrocephalus. MATERIALS AND METHODS: A total of 134 patients with hydrocephalus underwent MR imaging examination with a 3T unit consisting of turbo spin-echo, 3D-CISS, and cine phase-contrast (cine PC) sequences. 3D-CISS was used to assess obstructive membranes in CSF pathways compared with other sequences. Cine PC, follow-up imaging, and surgical findings were used to confirm obstructive membranes. RESULTS: Comparing the number of noncommunicating cases by using the conventional and 3D-CISS images, we found 26 new cases (19.4%) of 134 cases that were previously misdiagnosed as communicating hydrocephalus by conventional images. 3D-CISS sequence identified obstructive membranes invisible in other sequences, which facilitated selection of neuroendoscopy in the treat- ment of 31 patients (23.1%) in total who would have been otherwise treated with shunt insertion. These patients included 26 newly diagnosed noncommunicating cases after demonstration of intra- ventricular and/or fourth ventricular outlet membranes and 5 cases of communicating hydrocephalus with obstructing cisternal membranes. There were obstructions of the foramina of Luschka in 22 of 26 newly found noncommunicating cases. -

Anatomy and Physiology of Cerebrospinal Fluid
European Annals of Otorhinolaryngology, Head and Neck diseases (2011) 128, 309—316 Available online at www.sciencedirect.com REVIEW OF THE LITERATURE Anatomy and physiology of cerebrospinal fluid L. Sakka a,b,∗, G. Coll b, J. Chazal a,b a Laboratoire d’anatomie, faculté de médecine, université d’Auvergne, 28, place Henri-Dunant, 63001 Clermont-Ferrand cedex 1, France b Image-Guided Clinical Neuroscience and Connectomics, université d’Auvergne, UFR Médecine, CHU de Clermont-Ferrand, Hôpital Gabriel Montpied, 58 rue Montalembert, 63003 Clermont-Ferrand, France Available online 18 November 2011 KEYWORDS Summary The cerebrospinal fluid (CSF) is contained in the brain ventricles and the cranial Cerebrospinal fluid; and spinal subarachnoid spaces. The mean CSF volume is 150 ml, with 25 ml in the ventricles CSF; and 125 ml in subarachnoid spaces. CSF secretion; CSF is predominantly, but not exclusively, secreted by the choroid plexuses. Brain interstitial CSF circulation; fluid, ependyma and capillaries may also play a poorly defined role in CSF secretion. CSF space CSF circulation from sites of secretion to sites of absorption largely depends on the arterial comparative anatomy pulse wave. Additional factors such as respiratory waves, the subject’s posture, jugular venous pressure and physical effort also modulate CSF flow dynamics and pressure. Cranial and spinal arachnoid villi have been considered for a long time to be the predominant sites of CSF absorption into the venous outflow system. Experimental data suggest that cranial and spinal nerve sheaths, the cribriform plate and the adventitia of cerebral arteries constitute substantial pathways of CSF drainage into the lymphatic outflow system. -

Choroid Plexus of the Fourth Ventricle: Review and Anatomic Study Highlighting Anatomical Variations
Choroid Plexus of the Fourth Ventricle: Review and Anatomic Study Highlighting Anatomical Variations R. Shane Tubbs1, Mohammadali M. Shoja2, Anjali Aggarwal3, Tulika Gupta3, Marios Loukas4, Daisy Sahni3, Shaheryar F. Ansari5, Aaron A. Cohen-Gadol5 1 Seattle Science Foundation, Seattle, WA, USA 2 Pediatric Neurosurgery, Children’s of Alabama, Birmingham, AL, USA 3 Department of Anatomy, Postgraduate Institute of Medical Education and Research, Chandigarh India 4 Department of Anatomical Sciences, St. George’s University, Grenada 5 Goodman Campbell Brain and Spine, Indiana University Department of Neurological Surgery, Indianapolis, IN, USA Corresponding Author: Aaron A. Cohen-Gadol, MD, MSc Goodman Campbell Brain and Spine Indiana University, Department of Neurosurgery 355 W 16th St, Suite 5100 Indianapolis, IN 46202 E-mail: [email protected] Phone: 317-362-8760 Fax: 317-924-8472 __________________________________________________________________________________________ This is the author's manuscript of the article published in final edited form as: Tubbs, R. S., Shoja, M. M., Aggarwal, A., Gupta, T., Loukas, M., Sahni, D., … Cohen-Gadol, A. A. (2016). Choroid plexus of the fourth ventricle: Review and anatomic study highlighting anatomical variations. Journal of Clinical Neuroscience, 26, 79–83. http://dx.doi.org/10.1016/j.jocn.2015.10.006 1 Abstract Relatively few studies have been performed that comment on the morphology of the choroid plexus of the fourth ventricle. With this tissue’s importance as a landmark on imaging and during surgical intervention of the fourth ventricle, the authors performed a cadaveric study to better characterize this important structure. The choroid plexus of the fourth ventricle of sixty formalin fixed adult human brains was observed and measured. -

The Lateral Ventricles: a Detailed Review of Anatomy, Development, and Anatomic Variations
REVIEW ARTICLE The Lateral Ventricles: A Detailed Review of Anatomy, Development, and Anatomic Variations C.L. Scelsi, T.A. Rahim, J.A. Morris, G.J. Kramer, B.C. Gilbert, and S.E. Forseen ABSTRACT SUMMARY: The cerebral ventricles have been studied since the fourth century BC and were originally thought to harbor the soul and higher executive functions. During the infancy of neuroradiology, alterations to the ventricular shape and position on pneumo- encephalography and ventriculography were signs of mass effect or volume loss. However, in the current era of high-resolution cross-sectional imaging, variation in ventricular anatomy is more easily detectable and its clinical significance is still being investi- gated. Interpreting radiologists must be aware of anatomic variations of the ventricular system to prevent mistaking normal variants for pathology. We will review of the anatomy and development of the lateral ventricles and discuss several ventricular variations. he cerebral ventricles were the center of attention among phi- ventricular size and morphology but have been studied exten- Tlosophers, priests, anatomists, and physicians as far back as sively and will be left out of this review. Aristotle in the fourth century BC.1 They were originally thought to harbor the soul and “vital” spirits responsible for higher func- ANATOMY tions. After the influence of Christianity and the Renaissance, the Lateral Ventricles ventricles were conceptualized as 3 cavities where common sense, The lateral ventricles are paired C-shaped structures comprising creative imagination, and memory were individually allocated. It a body and atrium along with 3 projections into the frontal, tem- was not until the 16th century that anatomists Andreas Vesalius poral, and occipital lobes, termed “horns.” The lateral ventricles and Constanzo Varolio identified ventricles as being filled with communicate with the third ventricle through the interventricu- 2 CSF. -

The Formation and Function of the Brain Ventricular System
The Formation and Function of the Brain Ventricular System By Jessica T. Chang B.S. Molecular and Cellular Biology The Johns Hopkins University, 2006 Submitted to the Department of Biology in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology at the Massachusetts Institute of Technology June 2012 © 2012 Jessica T. Chang. All rights reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part. Signature of author……………………………………………………………………………………………………………………… Department of Biology May 4, 2012 Certified by…..…………………………………………………………………………………………………………………………….. Hazel L. Sive Professor of Biology Associate Dean, School of Science Thesis Supervisor Accepted by …………………………………………………………..…………………………………………………………………… Robert T. Sauer Salvador E. Luria Professor of Biology Chairman, Graduate Student Committee Chang | 1 Chang | 2 The Formation and Function of the Brain Ventricular System By Jessica T. Chang Submitted to the Department of Biology on April 13, 2012 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology at the Massachusetts Institute of Technology ABSTRACT The brain ventricular system is composed of a highly conserved set of cavities that contain cerebrospinal fluid (CSF), a protein-rich fluid essential for brain function. However, little is known about the function of embryonic CSF (eCSF), or the mechanisms of CSF production, retention, and circulation that regulate brain ventricle shape and size. Here we present data that begins to dissect the mechanisms governing CSF dynamics during zebrafish embryonic development. Our data indicate that the Na,K-ATPase regulates three aspects of brain ventricle development essential for normal function - neuroepithelial formation, permeability, and CSF production.