(Ug/M3) Long-Term ESL (Ppb) Date Derived Status Short
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improved Procedure for the Reductive Acetylation of Acyclic Esters and a New Synthesis of Ethers
J. Org. Chem. 2000, 65, 191-198 191 Improved Procedure for the Reductive Acetylation of Acyclic Esters and a New Synthesis of Ethers David J. Kopecky and Scott D. Rychnovsky* Department of Chemistry, University of California, Irvine, California 92717-2025 Received September 14, 1999 An optimized protocol for the DIBALH reductive acetylation of acyclic esters and diesters is described. This reductive acetylation procedure allows a wide variety of esters to be converted into the corresponding R-acetoxy ethers in good to excellent yields. It was found that, under mild acidic conditions, many R-acetoxy ethers can be further reduced to the corresponding ethers. This net two-step ester deoxygenation is an attractive alternative to the classical Williamson synthesis for certain ethers. Introduction The reduction and in situ acetylation of esters was developed in our laboratory several years ago to provide access to unusual cyclic acetal structures.1 The general strategy involved trapping of the aluminum hemiacetal intermediate found in the reduction of an ester to an aldehyde by diisobutylaluminum hydride (DIBALH). Other groups had previously trapped the same type of intermediate with a trimethylsilyl group.2 After some exploration, we found that acetic anhydride, DMAP, and pyridine led to efficient trapping to give R-acetoxy ethers 1 Figure 1. Original DIBALH reductive acetylation conditions from the cyclic esters we had been studying. We were for lactones and some acyclic esters. surprised to find that the same conditions gave satisfac- R tory yields of the -acetoxy ethers from acyclic esters. a 12 h period, an R-acetoxy ether of the general structure The R-acetoxy ethers can be activated with a variety of 3 was isolated. -

Thesis, Dissertation
THE COENZYME M BIOSYNTHETIC PATHWAY IN PROTEOBACTERIUM XANTHOBACTER AUTOTROPHICUS PY2 by Sarah Eve Partovi A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biochemistry MONTANA STATE UNIVERSITY Bozeman, Montana January 2018 ©COPYRIGHT by Sarah Eve Partovi 2018 All Rights Reserved ii DEDICATION I dedicate this dissertation to my family, without whom none of this would have been possible. My husband Ky has been a part of the graduate school experience since day one, and I am forever grateful for his support. My wonderful family; Iraj, Homa, Cameron, Shireen, Kevin, Lin, Felix, Toby, Molly, Noise, Dooda, and Baby have all been constant sources of encouragement. iii ACKNOWLEDGEMENTS First, I would like to acknowledge Dr. John Peters for his mentorship, scientific insight, and for helping me gain confidence as a scientist even during the most challenging aspects of this work. I also thank Dr. Jennifer DuBois for her insightful discussions and excellent scientific advice, and my other committee members Dr. Brian Bothner and Dr. Matthew Fields for their intellectual contributions throughout the course of the project. Drs. George Gauss and Florence Mus have contributed greatly to my laboratory technique and growth as a scientist, and have always been wonderful resources during my time in the lab. Members of the Peters Lab past and present have all played an important role during my time, including Dr. Oleg Zadvornyy, Dr. Jacob Artz, and future Drs. Gregory Prussia, Natasha Pence, and Alex Alleman. Undergraduate researchers/REU students including Hunter Martinez, Andrew Gutknecht and Leah Connor have worked under my guidance, and I thank them for their dedication to performing laboratory assistance. -

Evaluation of Liquid Ammonium Polyphosphate As a Carrier of Iron and Zinc
EVALUATION OF LIQUID AMMONIUM POLYPHOSPHATE AS A CARRIER OF IRON AND ZINC by ; V<P RATMUNDO RALLAN GANIRON B. S. A., University of the Philippines, 1961 A MASTER'S THESIS submitted in partial fulfillment of the requirement for the degree MASTER OF SCIENCE Department of Agronomy KANSAS STATE UNIVERSITY Manhattan, Kansas 1966 Approved by: Jor Professor LP ZGGf it Tj( £/<?7 TABLE OF CONTENTS INTRODUCTION 1 REVIEW OF LITERATURE 3 Iron . 3 Zinc * MATERIALS AND METHODS 13 Fertiliser Materials 13 Field Experiments H Soil Sampling 20 Laaf Sampling 22 Chemical Analysis 22 (a) Zinc 22 (b) Iron 24 (c) Phosphorua 25 (d) Potassium •« 27 (a) Organic Matter 27 (f) pH 27 (g) Protain 27 RESULTS AND DISCUSSIONS 26 SUMMARY AND CONCLUSIONS 4$ ACKNOWLEDGMENT. 51 LITERATURE CITED 52 INTRODUCTION Increasing attention has baan focused to the need of fertilising with micronutrient elements to achieve maximum production and optimum quality of farm crops. The supply of these elements in the soil has become limited in some areas as a result of the introduction of high yielding varieties, better and more intensive cropping practices, and increased use of high analysis fertilisers. In some cases, the ob- served deficiencies of these micronutrients have been man- made due to addition of interfering elements for other pur- poses. For instance there is the copper induced iron chlorosis, copper being introduced as agricultural sprays and the phosphorus induced iron and zinc deficiencies, phos- phorus being introduced to meet the phosphorus requirement of farm crops. How to minimise such interactions and the problems of keeping these micronutrient elements available to the plants when applied to the soil have been the sub- ject of intensive researches. -

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -

Synthesis and Antitumor Activity of Some Novel Thiophene, Pyrimidine, Coumarin, Pyrazole and Pyridine Derivatives
Acta Pharm. 67 (2017) 15–33 Original research paper DOI: 10.1515/acph-2017-0004 Synthesis and antitumor activity of some novel thiophene, pyrimidine, coumarin, pyrazole and pyridine derivatives MOHAMMED ALBRATTY1 2-Cyano-N-(thiazol-2-yl) acetamide (2a) and 2-cyano-N-(oxazol- 1,2 KARAM AHMED EL-SHARKAWY * 2-yl) acetamide (2b) were obtained via the reaction of ethyl cya- SHAMSHER ALAM1 noacetate with either 2-aminothiazole (1a) or 2-aminooxazole 1 Department of Pharmaceutical (1b). The formed products were directed toward the reaction Chemistry, College of Pharmacy with cyclopentanone and elemental sulfur in the presence of Jazan University, P.O. Box 114 triethylamine to give cyclopenta[b]thiophene derivatives (3a,b). Jazan 45142, Saudi Arabia The latter products were reacted with either ethyl cyanoacetate or malononitrile to form compounds 4a,b and 5a,b, respectively. 2 Department of Organic Chemistry Compounds 4a,b were aimed at synthesizing some heterocyclic Faculty of Biotechnology compounds; thus internal cyclization reactions were intro- October University for Modern duced to form compounds 6a,b. Also, compounds 4a,b reacted Sciences and Arts (MSA) with salicylaldehyde, hydrazine derivatives and either urea or El-Wahat Road thiourea to produce coumarin derivatives (7a,b), pyrazole de- 6 October City, Egypt rivatives (8a-d) and pyrimidine derivatives (9a-d), respectively. Reaction of either benzaldehyde or benzene diazonium chlo- ride (11) with compounds 4a,b afforded compounds 10a,b and 12a,b, respectively. On the other hand, compounds 5a,b under- went internal cyclization to form pyrimidine derivatives 13a,b. Also, when compounds 5a,b reacted with either ethyl cyanoac- etate or malononitrile, they gave pyridine derivatives (15a-d) through the formation of intermediates (14a-d). -

Synthesis of the Caffeine Metabolites 5-Acetylamino-6-Formylamino- 3-Methyluracil (AFMU) and 5-Acetylamino-6-Amino-3-Methyluracil (AAMU) on a Preparative Scale R
Synthesis of the Caffeine Metabolites 5-Acetylamino-6-formylamino- 3-methyluracil (AFMU) and 5-Acetylamino-6-amino-3-methyluracil (AAMU) on a Preparative Scale R. Röhrkasten3, P. Raatz3, R. P. Kreher3*, M. Blaszkewiczb a Lehrstuhl für Organische Chemie II, Fachbereich Chemie, Universität Dortmund. D-44227 Dortmund b Institut für Arbeitsphysiologie an der Universität Dortmund, ZWE Analytische Chemie, Ardeystraße 67, D-44139 Dortmund Z. Naturforsch. 52b, 1526-1532 (1997); received April 7, 1995 Pyrimidine-diones, Caffeine Metabolites, Synthesis 5-Acetylamino-6-amino-3-methyluracil (AAMU) and 5-acetylamino-6-formylamino-3- methyluracil (AFMU) have been prepared by simple chemical transformations starting from thiourea and ethyl cyanoacetate. These compounds AAMU and AFMU are required as standard materials for qualitative identification and quantitative determination in connection with the metabolism of caffeine. Introduction procedures are applied to obtain weighable amounts. The first method consisted in the con The N-acetyltransferase plays an important role sumption of a caffeinated beverage and the extrac in the metabolism of many xenobiotics; the pro tion of the metabolites from the urine; but the duction of the enzyme is genetically controlled. In yields are low [10]. A very expensive synthetic dividuals differ in the amount of the acetylated procedure is based on a procedure of Khmelevskii metabolites and can be classified as slow or rapid et al. [3] modified by Tang et al. [10] using 1-MU acetylators. The acetylator status is connected with instead of uric acid. After acylation with formic some diseases such as diabetes mellitus and blad acid/acetic anhydride the yield was only 19% pro der cancer and the knowledge of it is for the bene ducing 2 mg of AFMU 9. -

United States Patent ‘ Patented Mar
r 2,786,869 United States Patent ‘ Patented Mar. 26, 1957 l 2 tically, mixtures of tert-alkylamine such as are available on the market. Typical mixtures are those containing ‘ 2,786,869 C12H25NH2 to C15H31NH2 or C18H3'INH2 to C24H49NH2 N-TRIALKYLCARBINYL-N-(HYDROXYETHYL or C15H31- to C24H49NH2. These may be represented by POLYOXYETHYL) GLYCINES the formula Peter L. de Benneville and Homer J. Sims, Philadelphia, 31 Pa., assignors to Rohrn & Haas Company, Philadelphia, R’-—-C—NH2 Pa., a corporation of Delaware Rs No Drawing. Application June 16, 1954, 10 As catalysts in the ?rst step of the process of this Serial No. 437,273 invention, wherein the hydroxyethyl group is introduced, 9 Claims.‘ (or. 260-534) there may be used any of the strong acids, such as hy drochloric, hydrobromic, sulfuric, arylsulfonic, alkanesul fonic, or phosphoric. The preferred amount of this cat This invention relatesto-compounds of the structure 15 alyst is 10 to 30 mole percent of the amine. With R1 '(CH2CH2O),.H ‘ , amines from 12 carbon atoms upward it is exceedingly di?icult to introduce more than one hydroxyethyl group in a tert-alkylamine molecule. Such amines‘yicld ?nal 3 \CHrCOOH products which have the desired balance of properties. ' wherein R1, R2, and R3 are alkyl groups containing a 20 The ?rst reaction with ethylene oxide is effected by total of 11 to 23 carbon atoms and n is an integer having bringing together ethylene oxide and tert-alkylamine, a value from 5 to about 50 or more, preferably 5 to 25. usually by passing ethylene oxide into amine and catalyst, These compounds may be called N-(trialkylcarbinyl)-N at temperatures from 0° to 180° C. -

Raw Materials for Cosmetics
Raw materials for cosmetics Sasol Performance Chemicals Raw materials for cosmetics About us Raw materials for cosmetics Register of product groups About us Register of product groups Sasol’s Performance Chemicals business unit markets a broad portfolio 1 Fats and oils/esters 16 of organic and inorganic commodity and speciality chemicals. Our Special esters ................................................... 16 business consists four key business divisions: Organics, Inorganics, Castor oil derivatives ........................................... 16 Wax and PCASG (Phenolics, Carbon, Ammonia and Speciality Gases). Others ........................................................... 16 About 6300 people (incl. employees from Regional Operating Hubs) 2 Fatty alcohols 18 – 21 in offices in 18 countries serve customers around the world with a Single fractions ................................................. 18 multi-faceted portfolio of state-of-the-art chemical products and Blends ........................................................... 20 solutions for a wide range of applications and industries. Monobranched Guerbet alcohols ............................... 20 Our key products include surfactants, surfactant intermediates, fatty alcohols, linear alkyl 3 Petrolatum 22 – 23 benzene (LAB), short-chain linear alpha olefins, ethylene, petrolatum, paraffin waxes, 4 Paraffin waxes 24 – 25 synthetic waxes, cresylic acids, high-quality carbon solutions as well as high-purity and ultra-high-purity alumina. Our speciality gases sub-division supplies -
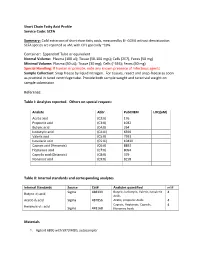
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -

Hydroxyacetonitrile (HOCH2CN) As a Precursor for Formylcyanide (CHOCN), Ketenimine (CH2CNH), and Cyanogen (NCCN) in Astrophysical Conditions
A&A 549, A93 (2013) Astronomy DOI: 10.1051/0004-6361/201219779 & c ESO 2013 Astrophysics Hydroxyacetonitrile (HOCH2CN) as a precursor for formylcyanide (CHOCN), ketenimine (CH2CNH), and cyanogen (NCCN) in astrophysical conditions G. Danger1, F. Duvernay1, P. Theulé1, F. Borget1, J.-C. Guillemin2, and T. Chiavassa1 1 Aix-Marseille Univ, CNRS, PIIM UMR 7345, 13397 Marseille, France e-mail: [email protected] 2 Institut des Sciences Chimiques de Rennes, École Nationale Supérieure de Chimie de Rennes, CNRS, UMR 6226, Avenue du Général Leclerc, CS 50837, 35708 Rennes Cedex 7, France Received 8 June 2012 / Accepted 19 November 2012 ABSTRACT Context. The reactivity in astrophysical environments can be investigated in the laboratory through experimental simulations, which provide understanding of the formation of specific molecules detected in the solid phase or in the gas phase of these environments. In this context, the most complex molecules are generally suggested to form at the surface of interstellar grains and to be released into the gas phase through thermal or non-thermal desorption, where they can be detected through rotational spectroscopy. Here, we focus our experiments on the photochemistry of hydroxyacetonitrile (HOCH2CN), whose formation has been shown to compete with aminomethanol (NH2CH2OH), a glycine precursor, through the Strecker synthesis. Aims. We present the first experimental investigation of the ultraviolet (UV) photochemistry of hydroxyacetonitrile (HOCH2CN) as a pure solid or diluted in water ice. Methods. We used Fourier transform infrared (FT-IR) spectroscopy to characterize photoproducts of hydroxyacetonitrile (HOCH2CN) and to determine the different photodegradation pathways of this compound. To improve the photoproduct identifications, irradiations of hydroxyacetonitrile 14N and 15N isotopologues were performed, coupled with theoretical calculations. -

NON-HAZARDOUS CHEMICALS May Be Disposed of Via Sanitary Sewer Or Solid Waste
NON-HAZARDOUS CHEMICALS May Be Disposed Of Via Sanitary Sewer or Solid Waste (+)-A-TOCOPHEROL ACID SUCCINATE (+,-)-VERAPAMIL, HYDROCHLORIDE 1-AMINOANTHRAQUINONE 1-AMINO-1-CYCLOHEXANECARBOXYLIC ACID 1-BROMOOCTADECANE 1-CARBOXYNAPHTHALENE 1-DECENE 1-HYDROXYANTHRAQUINONE 1-METHYL-4-PHENYL-1,2,5,6-TETRAHYDROPYRIDINE HYDROCHLORIDE 1-NONENE 1-TETRADECENE 1-THIO-B-D-GLUCOSE 1-TRIDECENE 1-UNDECENE 2-ACETAMIDO-1-AZIDO-1,2-DIDEOXY-B-D-GLYCOPYRANOSE 2-ACETAMIDOACRYLIC ACID 2-AMINO-4-CHLOROBENZOTHIAZOLE 2-AMINO-2-(HYDROXY METHYL)-1,3-PROPONEDIOL 2-AMINOBENZOTHIAZOLE 2-AMINOIMIDAZOLE 2-AMINO-5-METHYLBENZENESULFONIC ACID 2-AMINOPURINE 2-ANILINOETHANOL 2-BUTENE-1,4-DIOL 2-CHLOROBENZYLALCOHOL 2-DEOXYCYTIDINE 5-MONOPHOSPHATE 2-DEOXY-D-GLUCOSE 2-DEOXY-D-RIBOSE 2'-DEOXYURIDINE 2'-DEOXYURIDINE 5'-MONOPHOSPHATE 2-HYDROETHYL ACETATE 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID 2-METHYLFLUORENE 2-METHYL-2-THIOPSEUDOUREA SULFATE 2-MORPHOLINOETHANESULFONIC ACID 2-NAPHTHOIC ACID 2-OXYGLUTARIC ACID 2-PHENYLPROPIONIC ACID 2-PYRIDINEALDOXIME METHIODIDE 2-STEP CHEMISTRY STEP 1 PART D 2-STEP CHEMISTRY STEP 2 PART A 2-THIOLHISTIDINE 2-THIOPHENECARBOXYLIC ACID 2-THIOPHENECARBOXYLIC HYDRAZIDE 3-ACETYLINDOLE 3-AMINO-1,2,4-TRIAZINE 3-AMINO-L-TYROSINE DIHYDROCHLORIDE MONOHYDRATE 3-CARBETHOXY-2-PIPERIDONE 3-CHLOROCYCLOBUTANONE SOLUTION 3-CHLORO-2-NITROBENZOIC ACID 3-(DIETHYLAMINO)-7-[[P-(DIMETHYLAMINO)PHENYL]AZO]-5-PHENAZINIUM CHLORIDE 3-HYDROXYTROSINE 1 9/26/2005 NON-HAZARDOUS CHEMICALS May Be Disposed Of Via Sanitary Sewer or Solid Waste 3-HYDROXYTYRAMINE HYDROCHLORIDE 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE