PRACTICE GUIDELINE for the Treatment of Patients with HIV/AIDS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What You Should Know About HIV and AIDS
WHAT YOU SHOULD KNOW ABOUT HIV & AIDS WHAT IS AIDS? AIDS is the Acquired Immune Deficiency Syndrome – a serious illness that makes the body unable to fight infection. A person with AIDS is susceptible to certain infections and cancers. When a person with AIDS cannot fight off infections, this person becomes ill. These infections can eventually kill a person with AIDS. WHAT CAUSES AIDS? The human immunodeficiency virus (HIV) causes AIDS. Early diagnosis of HIV infection is important! If you have been told that you have HIV, you should get prompt medical treatment. In many cases, early treatment can enhance a person’s ability to remain healthy as long as possible. Free or reduced cost anonymous and confidential testing with counseling is available at every local health department in Kentucky. After being infected with HIV, it takes between two weeks to three months before the test can detect antibodies to the virus. HOW IS THE HIV VIRUS SPREAD? Sexual contact (oral, anal, or vaginal intercourse) with an infected person when blood, pre-ejaculation fluid, semen, rectal fluids or cervical/vaginal secretions are exchanged. Sharing syringes, needles, cotton, cookers and other drug injecting equipment with someone who is infected. Receiving contaminated blood or blood products (very unlikely now because blood used in transfusions has been tested for HIV antibodies since March 1985). An infected mother passing HIV to her unborn child before or during childbirth, and through breast feeding. Receipt of transplant, tissue/organs, or artificial insemination from an infected donor. Needle stick or other sharps injury in a health care setting involving an infected person. -

International Guidelines on HIV/AIDS and Human Rights 2006 Consolidated Version
International Guidelines on HIV/AIDS and Human Rights 2006 Consolidated Version Second International Consultation on HIV/AIDS and Human Rights Geneva, 23-25 September 1996 Third International Consultation on HIV/AIDS and Human Rights Geneva, 25-26 July 2002 Organized jointly by the Office of the United Nations High Commissioner for Human Rights and the Joint United Nations Programme on HIV/AIDS OFFICE OF THE UNITED NATIONS HIGH COMMISSIONER FOR HUMAN RIGHTS Material contained in this publication may be freely quoted or reprinted, provided credit is given and a copy containing the reprinted material is sent to the Office of the United Nations High Commissioner for Human Rights, CH-1211 Geneva 10, and to UNAIDS, CH-1211 Geneva 27, Switzerland. The designations employed and the presentation of the material in this publication do not imply expression of any opinion whatsoever on the part of the Secretariat of the United Nations or UNAIDS concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Published jointly by the Office of the United Nations High Commissioner for Human Rights and the Joint United Nations Programme on HIV/AIDS. HR/PUB/06/9 UN PUBLICATION Sales No. E.06.XIV.4 ISBN 92-1-154168-9 © Joint United Nations Programme on HIV/AIDS (UNAIDS) 2006. All rights reserved. Publications produced by UNAIDS can be obtained from the UNAIDS Information Centre. Requests for permission to translate UNAIDS publications—whether for sale or for noncommercial distribution—should also be addressed to the Information Centre at the address below, or by fax, at +41 22 791 4187, or e-mail: [email protected]. -
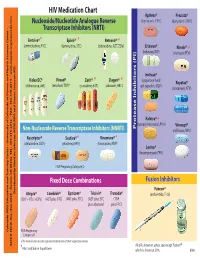
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (Tipranavir, TPV) (Darunavir, DRV) Transcriptase Inhibitors (NRTI)
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (tipranavir, TPV) (darunavir, DRV) Transcriptase Inhibitors (NRTI) Emtriva®* Epivir® * Retrovir® * (emtricitabine, FTC) (lamivudine, 3TC) (zidovudine, AZT, ZDV) Crixivan® Norvir® * (indinavir, IDV) (ritonavir, RTV) Invirase® Videx EC® Viread® Zerit® Ziagen® * * (saquinavir hard Reyataz® (didanosine, ddl) (tenofovir,TDF)* (stavudine, d4T) (abacavir, ABC) gel capsules, SQV) (atazanavir, ATV) Kaletra® * (lopinavir/ritonavir, LPV/r) Viracept® Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI) Inhibitors (PI) Protease (nelfinavir, NFV) Rescriptor® Sustiva®* Viramune® * (delavirdine, DLV) (efavirenz, EFV) (nevirapine, NVP) Lexiva® (fosamprenavir, FPV) FDA Pregnancy Category D Fixed Dose Combinations Fusion Inhibitors Fuzeon® Developed by MeriLou Johnson, MeriLou by Developed Johnson, MSW, and Steven MPA MD Atripla® Combivir® Epzicom® Trizivir® Truvada® (enfuvirtide,T-20) (TDF+FTC+EFV) (AZT plus 3TC) (ABC plus 3TC) (AZT plus 3TC (TDF plus abacavir) plus FTC) Colorado AIDS Education and Training Center • University of Colorado at Denver and Health Sciences Center • Center and Health Sciences Denver at of Colorado • University Center Training and AIDS Education Colorado FDA Pregnancy Category D ®The brands listed are the registered trademarks of their respective owners. 4200 E. Ave., Ninth TEL: A089 • Denver, Box 80262 CO 303-315-2512 • FAX: • 303-315-2514 • www.mpaetc.org/colorado.htm All pills shown in actual size except Fuzeon® *Also available in liquid form. which is shown at 50%. 8/06 Medication Schedule Name______________________________ Date___________ Number of Time of day you ar to take TOTAL Name of Medication pills to take this medicine Food Interactions Side Effects number of each time pills each day With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ Discontinued Medications or Formulations Helpful Hints: • Refill prescriptions before you run out. -

Pharmacokinetic Interactions Between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance
life Review Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance Laura Rombolà 1 , Damiana Scuteri 1,2 , Straface Marilisa 1, Chizuko Watanabe 3, Luigi Antonio Morrone 1, Giacinto Bagetta 1,2,* and Maria Tiziana Corasaniti 4 1 Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, Section of Preclinical and Translational Pharmacology, University of Calabria, 87036 Rende, Italy; [email protected] (L.R.); [email protected] (D.S.); [email protected] (S.M.); [email protected] (L.A.M.) 2 Pharmacotechnology Documentation and Transfer Unit, Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy 3 Department of Physiology and Anatomy, Tohoku Pharmaceutical University, 981-8558 Sendai, Japan; [email protected] 4 School of Hospital Pharmacy, University “Magna Graecia” of Catanzaro and Department of Health Sciences, University “Magna Graecia” of Catanzaro, 88100 Catanzaro, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0984-493462 Received: 28 May 2020; Accepted: 30 June 2020; Published: 4 July 2020 Abstract: The therapeutic efficacy of a drug or its unexpected unwanted side effects may depend on the concurrent use of a medicinal plant. In particular, constituents in the medicinal plant extracts may influence drug bioavailability, metabolism and half-life, leading to drug toxicity or failure to obtain a therapeutic response. This narrative review focuses on clinical studies improving knowledge on the ability of selected herbal medicines to influence the pharmacokinetics of co-administered drugs. Moreover, in vitro studies are useful to anticipate potential herbal medicine-drug interactions. -

Indinavir Sulfate Capsule Merck & Co., Inc
CRIXIVAN - indinavir sulfate capsule Merck & Co., Inc. ---------- CRIXIVAN® (INDINAVIR SULFATE) CAPSULES DESCRIPTION CRIXIVAN1 (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease. CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths of 100, 200, 333, and 400 mg of indinavir (corresponding to 125, 250, 416.3, and 500 mg indinavir sulfate, respectively). Each capsule also contains the inactive ingredients anhydrous lactose and magnesium stearate. The capsule shell has the following inactive ingredients and dyes: gelatin, titanium dioxide, silicon dioxide and sodium lauryl sulfate. The chemical name for indinavir sulfate is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-[2-[[(1,1 dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural formula: Indinavir sulfate is a white to off-white, hygroscopic, crystalline powder with the molecular formula C36H47N5O4• H2SO4 and a molecular weight of 711.88. It is very soluble in water and in methanol. 1 Registered trademark of MERCK & CO., Inc. COPYRIGHT © 1996, 1997, 1998, 1999, 2004 MERCK & CO., Inc. All rights reserved MICROBIOLOGY Mechanism of Action HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. -

Sexuality Education for Mid and Later Life
Peggy Brick and Jan Lunquist New Expectations Sexuality Education for Mid and Later Life THE AUTHORS Peggy Brick, M.Ed., is a sexuality education consultant currently providing training workshops for professionals and classes for older adults on sexuality and aging. She has trained thousands of educators and health care professionals nationwide, is the author of over 40 articles on sexuality education, and was formerly chair of the Board of the Sexuality Information and Education Council of the United States (SIECUS). Jan Lunquist, M.A., is the vice president of education for Planned Parenthood Centers of West Michigan. She is certified as a sexuality educator by the American Association of Sex Educators, Counselors, and Therapists. She is also a certified family life educator and a Michigan licensed counselor. During the past 29 years, she has designed and delivered hundreds of learning experiences related to the life-affirming gift of sexuality. Cover design by Alan Barnett, Inc. Printing by McNaughton & Gunn Copyright 2003. Sexuality Information and Education Council of the United States (SIECUS), 130 West 42nd Street, New York, NY 10036-7802. Phone: 212/819-9770. Fax: 212/819-9776. E-mail: [email protected] Web site: http://www.siecus.org 2 New Expectations This manual is dedicated to the memory of Richard Cross, M.D. 1915-2003 “What is REAL?” asked the Rabbit one day, when they were lying side by side near the nursery fender before Nana came to tidy the room. “Does it mean having things that buzz inside you and a stick-out handle?” “Real isn’t how you are made,” said the Skin Horse. -

CURRICULUM VITAE Updated on Jan 24, 2020
T. Davtyan, Armenia CURRICULUM VITAE Updated on Jan 24, 2020 Surname Davtyan First name(s) Tigran Affiliation and official address Rhea Pharmaceutical Armenia LLC Armenia, 0084, Yerevan Gusan Sherami St., 2 Building (Malatia-Sebastia adm. district) Republic of Armenia Tel.: (+374-) 91 400994, 98-55-96-29 E-mail: [email protected] [email protected] Present Positions: Professor, Chief Scientist Rhea Pharmaceutical Armenia LLC Home address: Michurin str. 5, 23, 375041, Yerevan, Republic of Armenia. Tel: +374 10 55-96-29 Date and Place of birth: 1 December, 1966, Yerevan, Armenia Nationality: Armenian Citizenship: Republic of Armenia Passport ARM, AH0248176, 24 OCT 2006, 009 ID № 1112660259 Marital status: Married, has 2 children, 3 grandchild Education (degrees, dates, universities) 2016 Professor. Field: Biology 2003 Doctor of Biological Sciences (ScD). Field: Molecular Biology 1993 Candidate of Biological Sciences (Ph.D); Field :Genetics and Immunology 1989-1992 Ph.D. student, Laboratory of Genetic Mechanisms of Cell Malignization And Differentiation, Institute of Cytology, St. Petersburg, Russia. 1983-1988 Yerevan State Medical University, Faculty of Pharmacy Specialization (specify) Drug Design and quality control, HIV/AIDS and clinical immunology; Viral infections; immunity and stress resistance; Molecular mechanisms and Genetic regulation of innate immune response; Autoinflammation; 1 T. Davtyan, Armenia Career/Employment (employers, positions and dates) 2011- 2019 Director of Analytical Laboratory of Scientific Centre of Drug and Medical Thechnology Experttise JSC 1999 - 2011 Head of HIV-Clinical Trail Laboratory of the ARMENICUM Research Center, Yerevan, Rep. of Armenia. 1998 - 2006 Consultant on Science, Laboratory of Immunology, The Second Clinical Hospital of the Yerevan State Medical University, Rep. -

HIV an Illusion Toxic Shock
SCIENTIFIC CORRESPONDENCE generated from a linear model could be a and Ho's] data is remarkable", note that vmons per day is not defined or function of both the baseline CD4 level Loveday et al. 8 report the use of a PCR addressed, nor can it be! No one else has and viral loads. Further studies with larg based assay and find only 200 HIV "virion access to either the unapproved drugs or er sample sizes are needed to resolve RNAs" per ml of serum of AIDS patients the branch PCR technology! What is the these discrepancies. - 1,000 times less than Ho and Wei. So benchmark rate for the turnover of CD4 Shenghan Lai, J. Bryan Page, Hong Lai much for the "remarkable concordance". cells in the general population? Departments of Medicine, Peter Duesberg To counter the 42 case studies of Wei et Psychiatry and Epidemiology, Department of Molecular and Cellular al. 1 and Ho et al.2, we at HEAL (Health University of Miami School of Medicine, Biology, University of California, Education AIDS Liason) can provide at Miami, Florida 33136, USA Berkeley, California 94720, USA least 42 people who are western-blot-posi Harvey Bialy tive for 'HIV', have low T4 cells, who are Bio/Technology, New York, not using orthodox procedures, and have HIV an illusion New York 10010, USA been healthy for years! On the other 1. Maddox, J. Nature 373, 189 (1995). hand, we can also provide you with hun SIR - In an editorial' in the 19 January 2. Ho, D.D. et al. Nature 373, 123-126 (1995). -
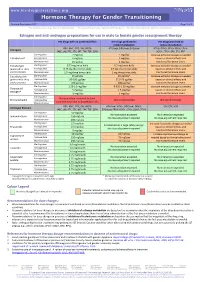
Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Estrogen and anti-androgen preparations for use in male to female gender reassignment therapy HIV drugs with no predicted effect HIV drugs predicted to HIV drugs predicted to inhibit metabolism induce metabolism RPV, MVC, DTG, RAL, NRTIs ATV/cobi, DRV/cobi, EVG/cobi ATV/r, DRV/r, FPV/r, IDV/r, LPV/r, Estrogens (ABC, ddI, FTC, 3TC, d4T, TAF, TDF, ZDV) SQV/r, TPV/r, EFV, ETV, NVP Starting dose 2 mg/day 1 mg/day Increase estradiol dosage as needed Estradiol oral Average dose 4 mg/day 2 mg/day based on clinical effects and Maximum dose 8 mg/day 4 mg/day monitored hormone levels. Estradiol gel Starting dose 0.75 mg twice daily 0.5 mg twice daily Increase estradiol dosage as needed (preferred for >40 y Average dose 0.75 mg three times daily 0.5 mg three times daily based on clinical effects and and/or smokers) Maximum dose 1.5 mg three times daily 1 mg three times daily monitored hormone levels. Estradiol patch Starting dose 25 µg/day 25 µg/day* Increase estradiol dosage as needed (preferred for >40 y Average dose 50-100 µg/day 37.5-75 µg/day based on clinical effects and and/or smokers) Maximum dose 150 µg/day 100 µg/day monitored hormone levels. Starting dose 1.25-2.5 mg/day 0.625-1.25 mg/day Increase estradiol dosage as needed Conjugated Average dose 5 mg/day 2.5 mg/day based on clinical effects and estrogen† Maximum dose 10 mg/day 5 mg/day monitored hormone levels. -

A History of the Hiv/Aids Epidemic with Emphasis on Africa *
UN/POP/MORT/2003/2 5 September 2003 ENGLISH ONLY WORKSHOP ON HIV/AIDS AND ADULT MORTALITY IN DEVELOPING COUNTRIES Population Division Department of Economic and Social Affairs United Nations Secretariat New York, 8-13 September 2003 A HISTORY OF THE HIV/AIDS EPIDEMIC WITH EMPHASIS ON AFRICA * UNAIDS and WHO ** * This document was reproduced without formal editing. ** UNAIDS, Geneva and WHO, Geneva. The views expressed in the paper do not imply the expression of any opinion on the part of the United Nations Secretariat. Quality and Coverage of HIV Sentinel Surveillance With a brief History of the HIV/AIDS Epidemic Workshop on HIV/AIDS and Adult Mortality in Developing Countries New York, 8-13 September 2003 2 1 History of the HIV/AIDS epidemic with emphasis on Africa In 1981, a new syndrome, the acquired immune deficiency syndrome (AIDS), was first recognized among homosexual men in the United States. By 1983, the etiological agent, the human immunodeficiency virus (HIV), had been identified. By the mid-1980’s, it became clear that the virus had spread, largely unnoticed, throughout most of the world. The HIV/AIDS pandemic consists of many separate epidemics. Each epidemic has its own distinct origin, in terms of geography and specific populations affected, and involve different types and frequencies of risk behaviors and practices, for example, unprotected sex with multiple partners or sharing drug injection equipment. Countries can be divided into three states: generalized, concentrated and low. Low Principle: Although HIV infection may have existed for many years, it has never spread to significant levels in any sub-population. -

Details of the Available Literature on Sex for Induction of Labour
Appendix 1: Details of the available literature on sex for induction of labour At term, nipple and genital stimulation have been advocated as a way of naturally promoting the release of endogenous oxytocin. 1 In 2005, a Cochrane Review examined the evidence for breast stimulation as a method for inducing labour and found six trials of 719 women, showing a decrease in the number of women not in labour at 72 hours with nipple stimulation compared with no intervention. 2 However, this finding was only significant among women who already had a favourable Bishop score (a cervical assessment used to predict the success of achieving a vaginal delivery). When breast stimulation was compared with intravenous oxytocin in the review, there was no difference in rates of cesarean delivery, number of women in labour at 72 hours or rates of meconium staining. However, the included studies did not look at time to vaginal delivery as an outcome. Overall, nipple stimulation seems to have minimal or no effect for women with an unripe cervix, but may be helpful for inducing labour in those with a ripe cervix. Few studies have looked at the role of intercourse as a cervical-ripening technique. However, prostaglandin concentrations have been shown to be 10 to 50 times higher in the cervical mucous of pregnant women two to four hours after intercourse, compared with concentrations before intercourse. 3 In a study of 47 women who had sex at term compared with 46 who abstained, there was no significant difference in Bishop scores. On average, the sexually active group delivered four days earlier, which was not considered clinically significant. -

(12) Patent Application Publication (10) Pub. No.: US 2008/0241135 A1 Olson Et Al
US 20080241135A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0241135 A1 Olson et al. (43) Pub. Date: Oct. 2, 2008 (54) METHODS FOR REDUCINGVIRAL LOAD IN 60/711,528, filed on Aug. 26, 2005, provisional appli HIV-1-INFECTED PATIENTS cation No. 60/715,619, filed on Sep. 9, 2005. (75) Inventors: William C. Olson, Ossining, NY Publication Classification (US); Paul J. Maddon, Scarsdale, NY (US); Daniel C. Pevear, (51) Int. Cl. Medford, MA (US); Robert J. A 6LX 39/395 (2006.01) Israel, Suffern, NY (US); Jose D. A6IP 43/00 (2006.01) Murga, Rosedale, NY (US) (52) U.S. Cl. ..................................................... 424/133.1 Correspondence Address: (57) ABSTRACT COOPER & DUNHAM, LLP 1185 AVENUE OF THE AMERICAS This method provides a method for reducing HIV-1 viral load NEW YORK, NY 10036 in an HIV-1-infected human subject which comprises admin istering to the subject at a predefined interval effective HIV-1 (73) Assignee: Progenics Pharmaceuticals, Inc. viral load-reducing doses of (a) a humanized antibody desig nated PRO 140, or of (b) an anti-CCR5 receptor monoclonal (21) Appl. No.: 11/894,762 antibody. This invention also provides a method for inhibiting in a human Subject the onset or progression of an HIV-1- (22) Filed: Aug. 20, 2007 associated disorder, the inhibition of which is effected by inhibiting fusion of HIV-1 to CCR5"CD4" target cells in the Related U.S. Application Data Subject. This invention also provides a method for treating a subject infected with HIV-1 comprising administering to the (63) Continuation of application No.