Carbonate-Hosted, Nonsulphide Zn (Hypogene) Mineral Deposit Profile E18
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of New Mineral Names: with an Index of Authors
415 A (fifth) list of new mineral names: with an index of authors. 1 By L. J. S~v.scs~, M.A., F.G.S. Assistant in the ~Iineral Department of the,Brltish Museum. [Communicated June 7, 1910.] Aglaurito. R. Handmann, 1907. Zeita. Min. Geol. Stuttgart, col. i, p. 78. Orthoc]ase-felspar with a fine blue reflection forming a constituent of quartz-porphyry (Aglauritporphyr) from Teplitz, Bohemia. Named from ~,Xavpo~ ---- ~Xa&, bright. Alaito. K. A. ~Yenadkevi~, 1909. BuU. Acad. Sci. Saint-P6tersbourg, ser. 6, col. iii, p. 185 (A~am~s). Hydrate~l vanadic oxide, V205. H~O, forming blood=red, mossy growths with silky lustre. Founi] with turanite (q. v.) in thct neighbourhood of the Alai Mountains, Russian Central Asia. Alamosite. C. Palaehe and H. E. Merwin, 1909. Amer. Journ. Sci., ser. 4, col. xxvii, p. 899; Zeits. Kryst. Min., col. xlvi, p. 518. Lead recta-silicate, PbSiOs, occurring as snow-white, radially fibrous masses. Crystals are monoclinic, though apparently not isom0rphous with wol]astonite. From Alamos, Sonora, Mexico. Prepared artificially by S. Hilpert and P. Weiller, Ber. Deutsch. Chem. Ges., 1909, col. xlii, p. 2969. Aloisiite. L. Colomba, 1908. Rend. B. Accad. Lincei, Roma, set. 5, col. xvii, sere. 2, p. 233. A hydrated sub-silicate of calcium, ferrous iron, magnesium, sodium, and hydrogen, (R pp, R',), SiO,, occurring in an amorphous condition, intimately mixed with oalcinm carbonate, in a palagonite-tuff at Fort Portal, Uganda. Named in honour of H.R.H. Prince Luigi Amedeo of Savoy, Duke of Abruzzi. Aloisius or Aloysius is a Latin form of Luigi or I~ewis. -

Revision 1 New Insights Into the Crystal Chemistry of Sauconite (Zn
This is the peer-reviewed, final accepted version for American Mineralogist, published by the Mineralogical Society of America. The published version is subject to change. Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2020-7460. http://www.minsocam.org/ Revision 1 New insights into the crystal chemistry of sauconite (Zn-smectite) from the Skorpion zinc deposit (Namibia) via a multi-methodological approach Emanuela Schingaro1*, Gennaro Ventruti1, Doriana Vinci1, Giuseppina Balassone2, Nicola Mondillo2, Fernando Nieto3, Maria Lacalamita1, Matteo Leoni4,5 1Dipartimento di Scienze della Terra e Geoambientali, Università degli Studi di Bari Aldo Moro, Via Orabona 4, I-70125, Bari, Italy 2Dipartimento di Scienze della Terra dell’Ambiente e delle Risorse, Università “Federico II”, Complesso Universitario Monte S. Angelo, Via Cintia, I-80126, Napoli, Italy 3Departamento de Mineralogía y Petrología, IACT, Universidad de Granada-CSIC, Av. Fuentenueva s/n, 18002, Granada, Spain 4Saudi Aramco Research and Development Center, P.O. Box 5000, 31311, Dhahran, Saudi Arabia 5Dipartimento di Ingegneria Civile, Ambientale e Meccanica, Università di Trento, Via Mesiano, 77, Trento, 38123, Italy *Corresponding author: Emanuela Schingaro, e-mail: [email protected] RUNNING TITLE: New insights into the crystal chemistry of sauconite 1 Always consult and cite the final, published document. See http:/www.minsocam.org or GeoscienceWorld This is the peer-reviewed, final accepted version for American Mineralogist, -

The Geochemistry and Mobility of Zinc in the Regolith. Advances in Regolith 2003 289
Advances in Regolith 2003 287 THE GEOCHEMISTRY AND MOBILITY OF ZINC IN THE REGOLITH D. C. McPhail1, Edward Summerhayes1, Susan Welch1 & Joël Brugger2 CRC LEME, Department of Geology, Australian National University, Canberra, ACT, 0200 1South Australian Museum and Adelaide University, Adelaide, SA 5000 INTRODUCTION The mobility of zinc in the regolith is important for several reasons, including the weathering of zinc deposits, formation of non-sulphide zinc deposits and contamination of soils and waters from human impact. The mobility of zinc is also important more generally to geologists and geochemists, both exploration and otherwise, because of the need to understand the formation of zinc ore deposits, such as Mississippi Valley Type (MVT), volcanic-hosted massive sulphide (VHMS), zinc oxide and others in which zinc occurs. This means that exploration geochemists, economic geologists and environmental scientists need to understand how zinc exists in the regolith, different lithologies and water, how it is mobilized or trapped, how far it can be transported and whether it is bioavailable and acts as either a micronutrient or a toxin to plant and animal life. In economic geology, there is presently an increasing interest in the formation of zinc oxide, or non- sulphide zinc deposits, and this is reflected in a recent special issue in the journal Economic Geology (Sangster 2003). Although the mobility of zinc in the regolith depends on the transporting process (e.g., groundwater advection or convection, sediment or airborne physical transport, biotic), it depends substantially on the geochemistry of zinc, i.e., how does zinc exist in groundwater and the regolith materials and what are the important geochemical reactions between water and solid. -

Supergene Mineralisation of the Boyongan Porphyry Copper-Gold Deposit, Surigao Del Norte, Philippines
Supergene Mineralisation of the Boyongan Porphyry Copper-Gold Deposit, Surigao del Norte, Philippines by Allan Maglaya Ignacio B.Sc. Geology, National Institute of Geological Sciences University of the Philippines Thesis submitted in partial fulfilment of the requirements of the Masters of Economic Geology Degree Centre for Ore Deposit Research, University of Tasmania December, 2005 DECLARATION OF ORIGINALITY This thesis contains no material which has been accepted for a degree of diploma by the University of Tasmania or any other institution, except by way of background information and duly acknowledged in the thesis, and contains no previous material previously pub- lished or written by another person except where due acknowledgement is given. Allan Maglaya Ignacio 01 December 2005 _________________________ STATEMENT OF AUTHORITY OF ACCESS This thesis may not to be made available for loan or copying for 1.5 years following the date this statement was signed. Following that time, the thesis may be available for loan and lim- ited copying in accordance with Copyright Act 1968. Allan Maglaya Ignacio 01 December 2005 _________________________ TABLE OF CONTENTS Page (s) LIST OF FIGURES …………………………………………………….. i - iii LIST OF APPENDICES ………………………………………………… iv ACKNOWLEDGMENTS ………………………………………………. v ABSTRACT ……………………………………………………………... vi - vii 1.0 INTRODUCTION ………………………………………………………. 1 - 8 1.1 Introduction …………………………………………………………. 1 1.2 Aims and Objectives ……………………………………………….. 1 1.3 Methods Employed …………………………………………………. 2 1.4 Location and Accessibility …………………………………………. 3 1.5 Climate ……………………………………………………………... 5 1.6 Previous Work ……………………………………………………… 5 2.0 GEOLOGICAL SETTING ………………………………………………. 9 - 37 2.1 Introduction ………………………………………………………. 9 2.2 Regional Tectonics …………….…………………………………. 9 2.3 Regional and Local Stratigraphy ………………………………... 11 2.3.1 Basement (Cretaceous-Paleogene) ………………………. 11 2.3.2 Bacuag Formation (Oliogocene-Miocene) .…………….. -

Zincite (Zn, Mn2+)O
Zincite (Zn, Mn2+)O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 6mm. Crystals rare, typically pyramidal, hemimorphic, with large {0001}, to 2.5 cm, rarely curved; in broad cleavages, foliated, granular, compact, massive. Twinning: On {0001}, with composition plane {0001}. Physical Properties: Cleavage: {1010}, perfect; parting on {0001}, commonly distinct. Fracture: Conchoidal. Tenacity: Brittle. Hardness = 4 VHN = 205–221 (100 g load). D(meas.) = 5.66(2) D(calc.) = 5.6730 Rare pale yellow fluorescence under LW UV. Optical Properties: Translucent, transparent in thin fragments. Color: Yellow-orange to deep red, rarely yellow, green, colorless; deep red to yellow in transmitted light; light rose-brown in reflected light, with strong red to yellow internal reflections. Streak: Yellow-orange. Luster: Subadamantine to resinous. Optical Class: Uniaxial (+). ω = 2.013 = 2.029 R1–R2: (400) 13.0–13.6, (420) 12.8–13.2, (440) 12.6–12.8, (460) 12.3–12.6, (480) 12.1–12.4, (500) 12.0–12.2, (520) 11.8–12.1, (540) 11.8–12.0, (560) 11.7–11.9, (580) 11.6–11.8, (600) 11.4–11.7, (620) 11.3–11.6, (640) 11.2–11.5, (660) 11.1–11.4, (680) 11.0–11.2, (700) 11.0–11.2 Cell Data: Space Group: P 63mc (synthetic). a = 3.24992(5) c = 5.20658(8) Z = 2 X-ray Powder Pattern: Synthetic. 2.476 (100), 2.816 (71), 2.602 (56), 1.626 (40), 1.477 (35), 1.911 (29), 1.379 (28) Chemistry: (1) (2) SiO2 0.08 FeO 0.01 0.23 MnO 0.27 0.29 ZnO 99.63 98.88 Total 99.99 [99.40] (1) Sterling Hill, New Jersey, USA. -

1 Vibrational Spectroscopic Characterization of the Phosphate
Vibrational spectroscopic characterization of the phosphate mineral phosphophyllite – Zn2Fe(PO4)2·4H2O, from Hagendorf Süd, Germany and in comparison with other zinc phosphates Ricardo Scholza, Ray L. Frost b, Yunfei Xi b, Leonardo M. Graçaa, Leonardo Lagoeiroa and Andrés López b a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane Queensland 4001, Australia. b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG, 35,400-00, Brazil Abstract: This research was undertaken on phosphophyllite sample from the Hagendorf Süd pegmatite, Bavaria, Germany. Chemical analysis was carried out by Scanning Electron Microscope in the EDS mode and indicates a zinc and iron phosphate with partial substitution of manganese, which partially replaced iron. The calculated chemical formula of the studied sample was -1 determined to be: Zn2(Fe0.65, Mn0.35)∑1.00(PO4)2·4(H2O). The intense Raman peak at 995 cm 3- is assigned to the ν1 PO4 symmetric stretching mode and the two Raman bands at 1073 and -1 3- 3- 1135 cm to the ν3 PO4 antisymmetric stretching modes. The ν4 PO4 bending modes are -1 3- -1 observed at 505, 571, 592 and 653 cm and the ν2 PO4 bending mode at 415 cm . The sharp Raman band at 3567 cm-1 attributed to the stretching vibration of OH units brings into question the actual formula of phosphopyllite. Vibrational spectroscopy enables an assessment of the molecular structure of phosphophyllite to be assessed. Key words: phosphophyllite, phosphate, pegmatite, Raman spectroscopy, infrared spectroscopy, Author to whom correspondence should be addressed ([email protected]) P +61 7 3138 2407 F: +61 7 3138 1804 1 Introduction Phosphophyllite is a rare Zn and Fe hydrous phosphate with general chemical formula expressed by Zn2Fe(PO4)2·4H2O [1, 2]. -

Download the Scanned
American Mineralogist, Volume 70, pages 379-387, 1985 Ma_nganesehumites and leucophoenicitesfrom Franklin and Sterling- Hill' NewJersev: 'i"? andimplications ;ifi':il1,;;lfiil11"r's' Perr J. Dullx Department of Mineral Sciences Smithsonian lnstitution, Washington, D. C. 20560 Abstract The manganesehumites, (alleghanyite, manganhumite, and sonolite),together with some Mn-bearing samplesof the Mg-humites,and the related phasesleucophoenicite and jerry- gibbsite,from the orebodiesat Franklin and SterlingHill, New Jersey,are describedtogether with analytical data. Solid solution betweenhumite and manganhumiteis at least partially continuous. Expected Mn/Mg solid solutions between alleghanyiteand chondrodite, and betweensonolite and clinohumite, are discontinuous; they are interrupted by apparently orderedphases. In all cases,the possibleorderings involve Zn as well as Mn and Mg. There are no Mn end-membersof the manganesehumites at this locality. Manganeseis apparently restricted in leucophoenicite(5.42-6.63 Mn per 7 octahedral cations) and in jerrygibbsite (7.79-8.02Mn per 9 octahedralcations). Calcium is common to both leucophoeniciteand jerrygibbsite,but among the Mn-humites,only sonoliteaccepts appreciable Ca (0.65Ca per 9 octahedralcations). There is a "threshold" level ofzinc in all studiedsamples; this "threshold" levelis a constantfor leucophoenicite1-9.3 Znper 3 Si)and alleghanyite(-O.2Zn per 2 Si). No samplesof leucophoeniciteor jerrygibbsite were found to be Zn-ftee,suggesting either that Zn is required for their stability, or that these two phasesmight not be stable as end-members.Fluorine is present in all the Mn-humites and is proportional to the Mg- content,but is absentin leucophoeniciteand jerrygibbsite. Introduction humite speciesoccur there; the Mg-humites occur in the The magnesiumhumite species(norbergite, chondrodite, host Franklin Marble for the most part, and the Mn- humite, and clinohumite) have been well-studiedand re- humites in the orebodiesthemselves. -

Leucophoenicite Mn (Sio4)3(OH)2
2+ Leucophoenicite Mn7 (SiO4)3(OH)2 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Crystals rare, typically slender, prismatic, elongated and striated [010], to 8 mm; in isolated grains or granular massive. Twinning: On k 001 , common, contact or interpenetrant twins, lamellar. f g Physical Properties: Cleavage: 001 , imperfect. Tenacity: Brittle. Hardness = 5.5{6 f g D(meas.) = 3.848 D(calc.) = [4.01] Optical Properties: Transparent to translucent. Color: Brown to light purple-red, raspberry-red, deep pink to light pink; rose-red to colorless in thin section. Luster: Vitreous. Optical Class: Biaxial ({). Pleochroism: Faint; rose-red 001 ; colorless 001 . Orientation: k f g ? f g X 001 cleavage. Dispersion: r > v; slight. ® = 1.751(3) ¯ = 1.771(3) ° = 1.782(3) ? f g 2V(meas.) = 74(5)± Cell Data: Space Group: P 21=a: a = 10.842(19) b = 4.826(6) c = 11.324(9) ¯ = 103:93(9)± Z = [2] X-ray Powder Pattern: Franklin, New Jersey, USA. 1.8063 (10), 2.877 (9), 2.684 (8), 4.36 (5), 3.612 (5), 2.365 (5), 2.620 (4) Chemistry: (1) (2) (3) (1) (2) (3) SiO2 26.36 26.7 26.7 CaO 5.67 2.4 2.8 FeO trace 0.3 0.3 Na2O 0.39 MnO 60.63 62.8 64.7 K2O 0.24 ZnO 3.87 0.0 0.0 H2O 2.64 [2.3] [2.8] MgO 0.21 5.5 2.7 Total 100.01 [100.0] [100.0] (1) Franklin, New Jersey, USA; composite of two analyses, corresponding to (Mn5:89Ca0:70Zn0:32 Na0:04Mg0:03K0:01)§=6:99(Si1:01O4)3(OH)2: (2) Kombat mine, Namibia; by electron microprobe, H2O by di®erence; corresponding to (Mn5:98Mg0:92Ca0:29Fe0:02)§=7:21(SiO4)3(OH)1:72: (3) Valsesia-Valtournanche area, Italy; by electron microprobe, H2O by di®erence; corresponding to (Mn6:16Mg0:45Ca0:34Fe0:03)§=6:98(SiO4)3(OH)2:10: Mineral Group: Leucophoenicite group. -

TEPHROITE from FRANKLIN, NEW JERSEY* Connbrrus S. Hunrsur
THE AMERICAN MINERALOGIST, VOL 46, MAY_JUNE, 1961 TEPHROITE FROM FRANKLIN, NEW JERSEY* ConNBrrus S. Hunrsur, Jn., Departmentof Mineralogy, Harvard, Uniaersity. Assrnlcr A study of tephroite specimens from Franklin and Sterling Hill, New Jersey showed in all of them the presence of thin sheets of willemite believed to be a product of exsolution. .fhese sheets are oriented parallel to the {100} and [010] planes of tephroite with the o and r axes of tephroite and willemite parallel. It is believed that Iittle zinc remains in the tephroite structure and that much of it reported in chemical analyses has been con- tributed by intergrown u'illemite. This conclusion is supported by experiments syn- thesizing tephroite. The indices of refraction and d spacing of {130} vary as would be expected with changes in amounts of MgO, FeO and CaO. INrnooucrroN 'fephroite, Mn2SiO4,a member of the olivine group, was describedas a new mineral from SterlingHill by Breithaupt in 1823.A chemicalanal- ysis of the original material was published by Brush (1864) together with severaladditional chemical analysesof tephroite made by others. These analysesreport ZnO in varying amounts which Brush attributed to invariably associatedzincite. Palache (1937) did not agree with Brush and stated " . that the molecularratios in someanalyses more nearly satisfy the orthosilicateformula when zinc is regardedas essen- tially a part of the mineral rather than as a constituent of mechanical inclusions." The present study was undertaken for the purpose of in- vestigatingthe variations in the propertiesof tephroite with changesin chemical composition, particularly the effect of zinc. Relationships were not expectedto be simplefor analysesshow, in addition to ZnO, variable amounts of MgO, FeO, and CaO. -

(Hypogene) Sulphate Minerals in Ore Deposits2
ECONOMIC GEOLOGY WITH WHICH IS INCORPORATED THE AMERICAN GEOLOGIST VOL. XIV DECEMBER, I9I 9 No. 8 PRIMARY (HYPOGENE) SULPHATE MINERALS IN ORE DEPOSITS2 B. S. BUTLER. CONTENTS. PAOE. Introduction ......................................................... Sulphate Minerals .................................................... Character of Ore Solutions as Indicated by Volcanic Emanations ....... 590 Conditions of Format'ion of Sulphur Trioxide ......................... 593 Formation of Sulphuric Acid in Nature ............................... 594 Formation of Primary Sulphate Minerals ............................. 596 Summary ............................................................ 6o8 Acknowledgment .................................. -................... 609 INTRODUCTION. In studyingore depositsthe writer has severaltimes been con.- fronted with the problemof accountingfor the formation of sulphateminerals that are apparentlyprimary. These minerals occur at placeswhere no evidencecan be found of their forma- tion by surface oxidation. Either they were formed directly 'by igneousemanations or, if they were formed by surfaceagencies, those agenciesmust have effectedthe depositionof other asso- ciatedminerals that would ordinarily •beregarded as of hypogene origin. An attemptwill be madeto analyzethis problemby start- ing with the belief, 'basedon deductionsfrom field observations, that the sulphatesin certaindeposits have •een formed directlyby x Published by permission of the Director, United State Geological Survey. 58x 5.82 B. S. BUTLER. -
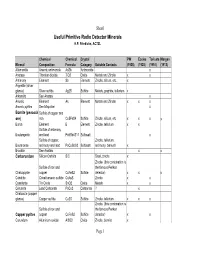
Useful Primitive Radio Detector Minerals H.P
Sheet1 Useful Primitive Radio Detector Minerals H.P. Friedichs, AC7ZL Chemical Chemical Crystal PW Eccles Toricata Morgan Mineral Composition Formula Category Suitable Contacts (1925) (1928) (1910) (1913) Allemontite Arsenic antimonide AsSb Antimonide x Anatase Titanium dioxide TiO2 Oxide Metals and Zincite x x Antimony Element Sb Element Zincite, silicon, etc. x Argentite (silver glance) Silver sulfide Ag2S Sulfide Metals, graphite, tellurium x Arkansite See Anatase x Arsenic Element As Element Metals and Zincite x x x Arsenic pyrites See Mispickel x Bornite (peacock Sulfide of copper and ore) iron Cu5FeS4 Sulfide Zincite, silicon, etc. x x x x Boron Element B Element Zincite, tellurium x x Sulfide of antimony Boulangerite and lead Pb5Sb4S11 Sulfosalt x Sulfide of copper, Zincite, tellurium, Bourmonite antimony and lead PbCuSbS3 Sulfosalt antimony, bismuth x Brookite See Anatase x x Carborundum Silicon Carbide SiC Steel, zincite x Zincite (this combination is Sulfide of iron and the famous Perikon Chalcopyrite copper CuFeS2 Sulfide detector) x x x Cobaltite Cobalt arsenic sulfide CoAsS Zincite x x Cassiterite Tin Oxide SnO2 Oxide Metals x x Cerussite Lead Carbonate PbCo3 Carbonate ?x Chalcocite (copper glance) Copper sulfide Cu2S Sulfide Zincite, tellurium x x x Zincite (this combination is Sulfide of iron and the famous Perikon Copper pyrites copper CuFeS2 Sulfide detector) x x Corundum Aluminum oxidde Al2O3 Oxide Zincite, bornite x Page 1 Sheet1 Covellite Copper sulfide CuS Sulfide Zincite, etc x x Cuprite (Cuprous oxide) Copper oxide -

Vibrational Spectroscopic Characterization of the Phosphate Mineral Phosphophyllite Â
Journal of Molecular Structure 1039 (2013) 22–27 Contents lists available at SciVerse ScienceDirect Journal of Molecular Structure journal homepage: www.elsevier.com/locate/molstruc Vibrational spectroscopic characterization of the phosphate mineral phosphophyllite – Zn2Fe(PO4)2Á4H2O, from Hagendorf Süd, Germany and in comparison with other zinc phosphates ⇑ Ricardo Scholz a, Ray L. Frost b, , Yunfei Xi b, Leonardo M. Graça a, Leonardo Lagoeiro a, Andrés López b a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35,400-00, Brazil highlights " Chemical analysis of phosphophyllite was carried out by Scanning Electron Microscope in the EDS mode. P " The chemical formula was determined to be: Zn2(Fe0.65,Mn0.35) 1.00(PO4)2Á4(H2O). " The mineral was characterized by both Raman and infrared spectroscopy. " An assessment of the molecular structure was made. " A comparison is made with the spectra of other zinc phosphate minerals. article info abstract Article history: This research was undertaken on phosphophyllite sample from the Hagendorf Süd pegmatite, Bavaria, Received 18 December 2012 Germany. Chemical analysis was carried out by Scanning Electron Microscope in the EDS mode and indi- Received in revised form 30 January 2013 cates a zinc and iron phosphate with partial substitution of manganese, which partially replaced iron. The Accepted 30 January 2013 P calculated chemical formula of the studied sample was determined to be: Zn2(Fe0.65,Mn0.35) 1.00(PO4)2- Available online 8 February 2013 À1 3À Á4(H2O).