Optical and Infrared Properties of the Atmosphere R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
SOLAR ENERGY Sun Is the Largest Source of Energy
SOLAR ENERGY Sun is the largest source of energy. Energy radiated from the sun is electromagnetic waves reaching the planet earth in three separated region, 1. Ultraviolet - 6.4 % (λ<0.38μm) 2. Visible -48 % (0.38 μm <λ<0.78μm) 3. Infrared -15.6 % (λ>0.78μm) When solar radiation (solar energy) is absorbed by a body it increases its energy. It provides the energy needed to sustain life in the solar system. It is a clean inexhaustible, abundantly and universally available energy and is the ultimate source of other various sources of energy. The heat generation is mainly due to various kinds of fusion reactions , the most of energy is released in which hydrogen combine to helium. An effective black body temperature of sun is 5777K Use of direct solar energy Solar thermal power plant Photolysis systems for fuel production Solar collector for water heating Passive solar heating system Photovoltaic, solar cell for electricity generation. Use of indirect solar energy Evaporation, precipitation, water flow Melting of snow Wave movements Ocean current Biomass production Heating of earth surface Wind Solar Constant: The rate at which solar energy arrives at the top of the atmosphere is called solar constant Isc Standard value of 1353 W/m2 adopted by NASA, but 1367 W/ m2, adopted by the world radiation center. Extra-terrestrial solar radiation: Solar radiation received on outer atmosphere of earth. Where, nth day of the year Terrestrial solar radiation: The solar radiation reaches earth surface after passing through the atmosphere is known as terrestrial solar radiation or global radiation. -

Contents 1 Properties of Optical Systems
Contents 1 Properties of Optical Systems ............................................................................................................... 7 1.1 Optical Properties of a Single Spherical Surface ........................................................................... 7 1.1.1 Planar Refractive Surfaces .................................................................................................... 7 1.1.2 Spherical Refractive Surfaces ................................................................................................ 7 1.1.3 Reflective Surfaces .............................................................................................................. 10 1.1.4 Gaussian Imaging Equation ................................................................................................. 11 1.1.5 Newtonian Imaging Equation.............................................................................................. 13 1.1.6 The Thin Lens ...................................................................................................................... 13 1.2 Aperture and Field Stops ............................................................................................................ 14 1.2.1 Aperture Stop Definition ..................................................................................................... 14 1.2.2 Marginal and Chief Rays ...................................................................................................... 14 1.2.3 Vignetting ........................................................................................................................... -

Air Mass Effect on the Performance of Organic Solar Cells
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 36 ( 2013 ) 714 – 721 TerraGreen 13 International Conference 2013 - Advancements in Renewable Energy and Clean Environment Air mass effect on the performance of organic solar cells A. Guechi1*, M. Chegaar2 and M. Aillerie3,4, # 1Institute of Optics and Precision Mechanics, Ferhat Abbas University, 19000, Setif, Algeria 2L.O.C, Department of Physics, Faculty of Sciences, Ferhat Abbas University, 19000, Setif, Algeria Email : [email protected], [email protected] 3Lorraine University, LMOPS-EA 4423, 57070 Metz, France 4Supelec, LMOPS, 57070 Metz, France #Email: [email protected] Abstract The objective of this study is to evaluate the effect of variations in global and diffuse solar spectral distribution due to the variation of air mass on the performance of two types of solar cells, DPB (etraphenyl–dibenzo–periflanthene) and CuPc (Copper-Phthalocyanine) using the spectral irradiance model for clear skies, SMARTS2, over typical rural environment in Setif. Air mass can reduce the sunlight reaching a solar cell and thereby cause a reduction in the electrical current, fill factor, open circuit voltage and efficiency. The results indicate that this atmospheric parameter causes different effects on the electrical current produced by DPB and CuPc solar cells. In addition, air mass reduces the current of the DPB and CuPc cells by 82.34% and 83.07 % respectively under global radiation. However these reductions are 37.85 % and 38.06%, for DPB and CuPc cells respectively under diffuse solar radiation. The efficiency decreases with increasing air mass for both DPB and CuPc solar cells. © 20132013 The The Authors. -

Chapter 19/ Optical Properties
Chapter 19 /Optical Properties The four notched and transpar- ent rods shown in this photograph demonstrate the phenomenon of photoelasticity. When elastically deformed, the optical properties (e.g., index of refraction) of a photoelastic specimen become anisotropic. Using a special optical system and polarized light, the stress distribution within the speci- men may be deduced from inter- ference fringes that are produced. These fringes within the four photoelastic specimens shown in the photograph indicate how the stress concentration and distribu- tion change with notch geometry for an axial tensile stress. (Photo- graph courtesy of Measurements Group, Inc., Raleigh, North Carolina.) Why Study the Optical Properties of Materials? When materials are exposed to electromagnetic radia- materials, we note that the performance of optical tion, it is sometimes important to be able to predict fibers is increased by introducing a gradual variation and alter their responses. This is possible when we are of the index of refraction (i.e., a graded index) at the familiar with their optical properties, and understand outer surface of the fiber. This is accomplished by the mechanisms responsible for their optical behaviors. the addition of specific impurities in controlled For example, in Section 19.14 on optical fiber concentrations. 766 Learning Objectives After careful study of this chapter you should be able to do the following: 1. Compute the energy of a photon given its fre- 5. Describe the mechanism of photon absorption quency and the value of Planck’s constant. for (a) high-purity insulators and semiconduc- 2. Briefly describe electronic polarization that re- tors, and (b) insulators and semiconductors that sults from electromagnetic radiation-atomic in- contain electrically active defects. -

Nikon Binocular Handbook
THE COMPLETE BINOCULAR HANDBOOK While Nikon engineers of semiconductor-manufactur- FINDING THE ing equipment employ our optics to create the world’s CONTENTS PERFECT BINOCULAR most precise instrumentation. For Nikon, delivering a peerless vision is second nature, strengthened over 4 BINOCULAR OPTICS 101 ZOOM BINOCULARS the decades through constant application. At Nikon WHAT “WATERPROOF” REALLY MEANS FOR YOUR NEEDS 5 THE RELATIONSHIP BETWEEN POWER, Sport Optics, our mission is not just to meet your THE DESIGN EYE RELIEF, AND FIELD OF VIEW The old adage “the better you understand some- PORRO PRISM BINOCULARS demands, but to exceed your expectations. ROOF PRISM BINOCULARS thing—the more you’ll appreciate it” is especially true 12-14 WHERE QUALITY AND with optics. Nikon’s goal in producing this guide is to 6-11 THE NUMBERS COUNT QUANTITY COUNT not only help you understand optics, but also the EYE RELIEF/EYECUP USAGE LENS COATINGS EXIT PUPIL ABERRATIONS difference a quality optic can make in your appre- REAL FIELD OF VIEW ED GLASS AND SECONDARY SPECTRUMS ciation and intensity of every rare, special and daily APPARENT FIELD OF VIEW viewing experience. FIELD OF VIEW AT 1000 METERS 15-17 HOW TO CHOOSE FIELD FLATTENING (SUPER-WIDE) SELECTING A BINOCULAR BASED Nikon’s WX BINOCULAR UPON INTENDED APPLICATION LIGHT DELIVERY RESOLUTION 18-19 BINOCULAR OPTICS INTERPUPILLARY DISTANCE GLOSSARY DIOPTER ADJUSTMENT FOCUSING MECHANISMS INTERNAL ANTIREFLECTION OPTICS FIRST The guiding principle behind every Nikon since 1917 product has always been to engineer it from the inside out. By creating an optical system specific to the function of each product, Nikon can better match the product attri- butes specifically to the needs of the user. -

The Ray Optics Module User's Guide
Ray Optics Module User’s Guide Ray Optics Module User’s Guide © 1998–2018 COMSOL Protected by patents listed on www.comsol.com/patents, and U.S. Patents 7,519,518; 7,596,474; 7,623,991; 8,457,932; 8,954,302; 9,098,106; 9,146,652; 9,323,503; 9,372,673; and 9,454,625. Patents pending. This Documentation and the Programs described herein are furnished under the COMSOL Software License Agreement (www.comsol.com/comsol-license-agreement) and may be used or copied only under the terms of the license agreement. COMSOL, the COMSOL logo, COMSOL Multiphysics, COMSOL Desktop, COMSOL Server, and LiveLink are either registered trademarks or trademarks of COMSOL AB. All other trademarks are the property of their respective owners, and COMSOL AB and its subsidiaries and products are not affiliated with, endorsed by, sponsored by, or supported by those trademark owners. For a list of such trademark owners, see www.comsol.com/trademarks. Version: COMSOL 5.4 Contact Information Visit the Contact COMSOL page at www.comsol.com/contact to submit general inquiries, contact Technical Support, or search for an address and phone number. You can also visit the Worldwide Sales Offices page at www.comsol.com/contact/offices for address and contact information. If you need to contact Support, an online request form is located at the COMSOL Access page at www.comsol.com/support/case. Other useful links include: • Support Center: www.comsol.com/support • Product Download: www.comsol.com/product-download • Product Updates: www.comsol.com/support/updates • COMSOL Blog: www.comsol.com/blogs • Discussion Forum: www.comsol.com/community • Events: www.comsol.com/events • COMSOL Video Gallery: www.comsol.com/video • Support Knowledge Base: www.comsol.com/support/knowledgebase Part number: CM024201 Contents Chapter 1: Introduction About the Ray Optics Module 8 The Ray Optics Module Physics Interface Guide . -

Transmittance of Optical Glass TIE-35
Technical Information 1 Advanced Optics Version November 2020 TIE-35 Transmittance of optical glass Introduction 1. Theoretical background ......................... 1 Optical glasses are optimized to provide excellent transmit- tance throughout the total visible range from 380 to 780 nm 2. Wavelength dependence of transmittance ....... 2 (perception range of the human eye). Usually the transmit- 3. Measurement and specification.................. 7 tance range spreads also into the near UV and IR regions. As a general trend lowest refractive index glasses show high trans- 4. Literature........................................ 9 mittance far down to short wavelengths in the UV. Going to higher index glasses the UV absorption edge moves closer to the visible range. For highest index glass and larger thickness past. And for special applications where best transmittance the absorption edge already reaches into the visible range. is required SCHOTT offers improved quality grades like for This UV-edge shift with increasing refractive index is explained SF57 the grade SF57HTultra. by the general theory of absorbing dielectric media. So it may not be overcome in general. However, due to improved The aim of this technical information is to give the optical melting technology high refractive index glasses are offered designer a deeper understanding on the transmittance prop- nowadays with better blue-violet transmittance than in the erties of optical glass. 1. Theoretical background A light beam with the intensity I0 falls onto a glass plate having The beam reflected at the exit surface returns to the entrance a thickness d (figure 1). At the entrance surface part of the surface and is divided into a transmitted and a reflected part. -

AIR MASS 2 SOLAR SIMULATOR CSCL 10A X (NASA) 10 P HC $3.25 Unclas G3/44 08986 Z
NASA TECHNICAL NASA TM X- 71662 MEMORANDUM (1J N75-16080 (NASA-TM-X-716 6 2) OPERATIONAL PERFORMANCE S OF A LOW COST, AIR MASS 2 SOLAR SIMULATOR CSCL 10A X (NASA) 10 p HC $3.25 Unclas G3/44 08986 z OPERATIONAL PERFORMANCE OF A LOW COST, 113141S7;, AIR MASS 2 SOLAR SIMULATOR " by Kenneth Yass and Henry B., Curtis e Lewis Research Center , Cleveland, Ohio 44135 6282P3 TECHNICAL PAPER to be presented at Institute of Environmental Sciences Conference Los Angeles, California, April 13-16, 1975 OPERATIONAL PERFORMANCE OF A LOW COST, AIR MASS 2 SOLAR SIMULATOR By: Kenneth Yass and Henry B. Curtis, NASA Lewis Research Center Kenneth Yass is in the Optical Measurements Section utilization of solar energy. Tests are being of the Instrument Development and Applications conducted both indoors and outdoors. Indoor tests Office at the Lewis Research Center. He started are made for a period of hours and are used to at NACA in 1950 with a BS in physics from CCNY, determine collector performance under ideal condi- and an MS from WVaU. Early assignments were in tions and are used to evaluate performance changes. the field of general instrumentation. When NACA Indoor tests are also very useful for making rapid became a NASA space center, he turned his atten- comparisons between several collectors. Outdoor tion to solar simulation for outer space. His most tests are conducted over much longer periods of recent activity has been solar simulation for time and can determine collector performance under utilization of solar energy. The Air Mass 2 changing weather conditions and prolonged use. -
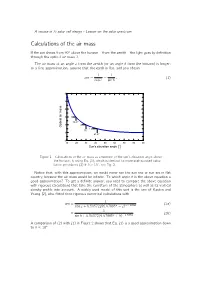
Calculations of the Air Mass
A course in Si solar cell design - Lesson on the solar spectrum Calculations of the air mass If the sun shines from 90± above the horizon { from the zenith { the light goes by de¯nition through the optical air mass 1. The air mass at an angle z from the zenith (or an angle h from the horizon) is longer: to a ¯rst approximation, assume that the earth is flat, and you obtain 1 1 am = = ; (1) cos z sin h 6 5 4 3 3 19.47° 2 2 Optical air mass 1.5 30° 1 41.81° 0 10 20 30 40 50 60 70 80 90 Sun's elevation angle [°] Figure 1 Calculations of the air mass as a function of the sun's elevation angle above the horizon, h, using Eq. (1), which is identical to more sophisticated calcu- lation procedures (2) if h > 10±, see Fig. 2. Notice that, with this approximation, we would never see the sun rise or sun set in flat country, because the air mass would be in¯nite. To which angle h is the above equation a good approximation? To get a de¯nite answer, you need to compare the above equation with rigorous calculations that take the curvature of the atmosphere as well as its vertical density pro¯le into account. A widely used model of this sort is the one of Kasten and Young [2], who ¯tted their rigorous numerical calculations with 1 am = (2a) cos z + 0:50572(96:07995± ¡ z)¡1:6364 1 = (2b) sin h + 0:50572(6:07995± + h)¡1:6364 A comparison of (2) with (1) in Figure 2 shows that Eq. -

Dispersion of Infrared Optical Materials
Dispersion properties of mid‐infrared optical materials Andrei Tokmakoff December 2016 Contents 1) Dispersion calculations for ultrafast mid‐IR pulses 2) Index of refraction of optical materials in the mid‐IR 3) Sellmeier equations 4) Second‐order dispersion (fs2 mm‐1) 5) Third‐order dispersion (fs3 mm‐1) 6) Zero group velocity dispersion wavelengths This material is offered as a resource for calculating the dispersion of ultrafast mid‐IR laser pulses through optical devices. For additional reading on this topic see: “The role of dispersion in ultrafast optics,” Ian Walmsley, Leon Waxer, and Christophe Dorrer, Review of Scientific Instruments 72, 1‐29 (2001). “Dispersion compensation with optical materials for compression of intense sub‐100 femtosecond mid‐ infrared pulses,” N. Demirdöven, M. Khalil, O. Golonzka and A. Tokmakoff, Optics Letters, 27, 433‐435 (2002). For refractive index data: “Handbook of Optical Constants of Solids,” Edited by Edward D. Palik, (Academic Press, San Diego, 1998) http://refractiveindex.info/ Dispersion calculations for short mid‐IR pulses The optical dispersion experienced by a short pulse propagating through an spectrometer is calculated in the frequency domain from the spectral phase ϕ(ω). The field is expressed as i EIe () The spectral phase is related to the frequency dependent optical pathlength P as P c and the optical pathlength is related to the index of refraction and geometric path length as Pn() ()() . Commonly, we choose to expand ϕ about the center frequency of the pulse spectrum ω0: 1 2 12 002 0 1 1 P P c 00 22 1 PP 2 2 22c 00 Here, the first order term ϕ(1) is inversely proportional to the group delay for the pulse and the second order term ϕ(2) is the group delay dispersion or group velocity dispersion (GVD). -

1 Solar Cell Efficiency Divergence Due to Operating Spectrum Variation
Published version: Solar Energy v. 217, Pages 49-57, 2021, doi.org/10.1016/j.solener.2021.01.024 Solar cell efficiency divergence due to operating spectrum variation Geoffrey S. Kinsey Zuva Energy Abstract A limitation in the performance rating of solar cells and modules is that they are tested using a single value for the solar spectrum. To map the performance expected under the varying spectra found in operating conditions, solar cell efficiencies have been evaluated using solar spectra generated by the National Solar Radiation Database, applied to confirmed record-efficiency cell parameters. Nine solar cell types (single-junction and multijunction) are evaluated using spectra at more than forty locations, spanning 76° of latitude and 150° of longitude, at hourly intervals over a year. Relative to the standard test efficiency, increases in annual operating efficiency are seen in cadmium telluride and single-junction perovskite designs, while efficiency decreases are observed in two-terminal multijunction structures. Though silicon exhibits the least variation, its -3% to +2% range in relative efficiency is equivalent to ~20° C of temperature variation. This divergence in operating efficiencies indicates that evaluation using a single spectrum is not a sufficient basis for comparison, or prediction of energy yield in operation. Application of an additional “operating spectrum,” to complement the standard test spectrum, is proposed. Fig. 1. Source locations for spectra in this study. Each marker represents about 4000 hourly spectra, for wavelengths from 280 nm to 4000 nm. In six locations, hourly spectra were generated for direct normal irradiance (DNI) using inputs from the Typical Meteorological Year 3 database (NREL, 2010) to generate spectra using the SMARTS model (Gueymard, 1995). -

Reflection and Refraction
Reflection and refraction Ivan Bazarov Cornell Physics Department / CLASSE Outline • Reflection & refraction in geometric optics • Fresnel equations • Applications 1 Reflection and refraction P3330 Exp Optics Fermat’s principle: reflection Fermat’s principle: optical path is stationary (has an extremum with Optical path = path × index of refraction respect to small changes) Law of reflection ⇥i =⇥r 2 Reflection and refraction P3330 Exp Optics Fermat’s principle: refraction Snell’s law n sin ⇥= n sin ⇥ ⇢ 0 0 ⇢0 SP = n⇢ + n0⇢0 SP + dSP = n(⇢ dx sin ⇥) + n0(⇢0 + dx sin ⇥0) f − dSP f =0=f n( sin ⇥) + n0 sin ⇥0 dx − f 3 Reflection and refraction P3330 Exp Optics Look at the problem as E&M wave at the interface • Geometric optics can provide no information about wave amplitudes • Need E&M (vector!) theory of light Perpendicular (“S”) polarization “sticks out” of or into the plane of ! Incident medium ! incidence. ki kr Ei Er n Plane of incidence i (here the xy plane) is qi qr the plane that contains InterfacePlane of the interface (here the yz the incident and plane)y (perpendicular to page) reflected k-vectors. qt x nt z E ! Parallel (“P”) t k polarization lies parallel Transmitting medium t to the plane of incidence. B-field not shown here, but it’s always perpendicular to E-field 4 Reflection and refraction P3330 Exp Optics Fresnel equations • Goal: relate amplitudes of transmitted and reflected waves • Fresnel was the first to obtain the expressions in early 1800’s. • A complication: must consider two polarizations separately S- and P- or ⊥ and || w.r.t.