Estimation of Water Vole Abundance by Using Surface Indices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mammal Extinction Facilitated Biome Shift and Human Population Change During the Last Glacial Termination in East-Central Europeenikő
Mammal Extinction Facilitated Biome Shift and Human Population Change During the Last Glacial Termination in East-Central EuropeEnikő Enikő Magyari ( [email protected] ) Eötvös Loránd University Mihály Gasparik Hungarian Natural History Museum István Major Hungarian Academy of Science György Lengyel University of Miskolc Ilona Pál Hungarian Academy of Science Attila Virág MTA-MTM-ELTE Research Group for Palaeontology János Korponai University of Public Service Zoltán Szabó Eötvös Loránd University Piroska Pazonyi MTA-MTM-ELTE Research Group for Palaeontology Research Article Keywords: megafauna, extinction, vegetation dynamics, biome, climate change, biodiversity change, Epigravettian, late glacial Posted Date: August 11th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-778658/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/27 Abstract Studying local extinction times, associated environmental and human population changes during the last glacial termination provides insights into the causes of mega- and microfauna extinctions. In East-Central (EC) Europe, Palaeolithic human groups were present throughout the last glacial maximum (LGM), but disappeared suddenly around 15 200 cal yr BP. In this study we use radiocarbon dated cave sediment proles and a large set of direct AMS 14C dates on mammal bones to determine local extinction times that are compared with the Epigravettian population decline, quantitative climate models, pollen and plant macrofossil inferred climate and biome reconstructions and coprophilous fungi derived total megafauna change for EC Europe. Our results suggest that the population size of large herbivores decreased in the area after 17 700 cal yr BP, when temperate tree abundance and warm continental steppe cover both increased in the lowlands Boreal forest expansion took place around 16 200 cal yr BP. -

Microtus Duodecimcostatus) in Southern France G
Capture-recapture study of a population of the Mediterranean Pine vole (Microtus duodecimcostatus) in Southern France G. Guédon, E. Paradis, H Croset To cite this version: G. Guédon, E. Paradis, H Croset. Capture-recapture study of a population of the Mediterranean Pine vole (Microtus duodecimcostatus) in Southern France. Mammalian Biology, Elsevier, 1992, 57 (6), pp.364-372. ird-02061421 HAL Id: ird-02061421 https://hal.ird.fr/ird-02061421 Submitted on 8 Mar 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Capture-recapture study of a population of the Mediterranean Pine vole (Microtus duodecimcostatus) in Southern France By G. GUEDON, E. PARADIS, and H. CROSET Laboratoire d'Eco-éthologie, Institut des Sciences de l'Evolution, Université de Montpellier II, Montpellier, France Abstract Investigated the population dynamics of a Microtus duodecimcostatus population by capture- recapture in Southern France during two years. The study was carried out in an apple orchard every three months on an 1 ha area. Numbers varied between 100 and 400 (minimum in summer). Reproduction occurred over the year and was lowest in winter. Renewal of the population occurred mainly in autumn. -

What Does the Oxygen Isotope Composition of Rodent Teeth Record?
Earth and Planetary Science Letters 361 (2013) 258–271 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl What does the oxygen isotope composition of rodent teeth record? Aure´lien Royer a,e, Christophe Le´cuyer a,f,n, Sophie Montuire b,e, Romain Amiot a, Serge Legendre a, Gloria Cuenca-Besco´ s c, Marcel Jeannet d, Franc-ois Martineau a a Laboratoire de Ge´ologie de Lyon, UMR CNRS 5276, Universite´ Lyon 1 et Ecole Normale Supe´rieure de Lyon, 69622 Villeurbanne, France b Bioge´osciences, UMR CNRS 5561, Universite´ de Bourgogne, 6 Boulevard Gabriel, 21000 Dijon, France c Departamento de Ciencias de la Tierra, A´rea de Paleontologı´a, Edificio de Geolo´gicas, 50009 Zaragoza, Spain d LAMPEA, UMR 6636, MMSH, 5 rue du Chateauˆ de l’Horloge, BP 647, 13094 Aix-en-Provence Cedex 2, France e Laboratoire Pale´obiodiversite´ et Evolution, EPHE-Ecole Pratique des Hautes Etudes, 21000 Dijon, France f Institut Universitaire de France, Paris, France article info abstract 18 Article history: Oxygen isotope compositions of tooth phosphate (d Op) were measured in 107 samples defined on the Received 6 December 2011 basis of teeth obtained from 375 specimens of extant rodents. These rodents were sampled from pellets Received in revised form collected in Europe from 381N (Portugal) to 651N (Finland) with most samples coming from sites 25 September 2012 18 located in France and Spain. Large oxygen isotopic variability in d Op is observed both at the intra- and Accepted 28 September 2012 inter-species scale within pellets from a given location. -

Swimming Ability in Three Costa Rican Dry Forest Rodents
Rev. Biol. Trop. 49(3-4): 1177-1181, 2001 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Swimming ability in three Costa Rican dry forest rodents William M. Cook1, Robert M. Timm1 and Dena E. Hyman2 1 Department of Ecology and Evolutionary Biology & Natural History Museum, Dyche Hall, University of Kansas, Lawrence, KS 66045 USA. Fax: (785) 864-5335. E-mail: [email protected]. 2 Department of Biology, University of Miami, Coral Gables, FL 33124 USA. Received 18-VII-2000. Corrected 20-III-2001. Accepted 30-III-2001. Abstract: We investigated the swimming abilities of three Costa Rican dry forest rodents (Coues’ rice rat, Oryzomys couesi, hispid cotton rat, Sigmodon hispidus, and spiny pocket mouse, Liomys salvini) associated with a large marsh, Laguna Palo Verde, using 90 s swim trials in a plastic container. Swimming ability was evaluated by observing the use of limbs and tail in the water, inclination to the surface, and diving and float- ing behavior. Rice rats could float, swim and dive, suggesting that they can exploit surface and underwater resources. Cotton rats swam at the water’s surface, but were less skilled swimmers than rice rats. Spiny pocket mice tired quickly and had difficulty staying at the water’s surface. Results suggest that differential swimming ability is related to the distribution of the three sympatric species within the marsh and adjacent forest habitats. Key words: Dry forest, Liomys salvini, Oryzomys couesi, Sigmodon hispidus, swimming ability. Most rodents can swim when necessary, level, which in recent years has been domina- and may even dive and swim skillfully (Dagg ted by dense stands of emergent cattails and Windsor 1972). -
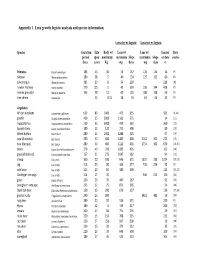
Appendix 1. Lens Growth Logistic Analysis and Species Information
Appendix 1. Lens growth logistic analysis and species information. Lens dry wt logistic Lens wet wt logistic Species Gestation Life Body wt Lens wt Lens wt Lenses Data period span maximum maximum Slope maximum Slope or data source days years Kg mg days mg days n Primates Papio hamadryas 183 45 30 55 167 178 124 16 PS baboon Macaca fascicularis 180 38 5 40 130 125 162 60 PS cynomolgus Alouatta caraya 185 20 8 34 220 228 [8] howler monkey Homo sapiens 270 115 5 45 359 198 244 608 PS human prenatal Macaca mulatta 165 38 12 60 125 188 108 50 PS tree shrew Tupaia glis 12 0.15 28 76 63 63 32 PS Ungulates African elephant Loxodonta a africana 659 80 5000 475 935 561 [9,10] giraffe Giraffa camelopardalis 460 35 2000 1163 571 34 [11] hipppotamus Hippopotamus amphibius 250 55 3000 410 362 360 [12] Spanish Ibex Capra pyrenaica Schinz 180 12 120 711 508 80 [13] Wood buffalo Bison bison 280 25 1000 1288 525 97 [14] cow (Australia) Bos taurus 280 30 600 1483 400 3162 302 231 [15] cow (Europe) Bos taurus 280 30 400 1122 401 2754 302 630 [16,17] zebra Equus burchelli antiquorum 370 40 350 1085 436 102 [18] gnu (wildebeest) Connochaetes taurinus 257 21 275 1047 362 94 [19] sheep Ovis aries 160 20 100 646 311 1622 239 1224 [20,21] pig Sus scrofa 115 25 80 364 277 736 254 92 PS wild boar Sus scrofa 115 20 84 389 309 113 [22,23] Gottingen minipigs Sus scrofa 114 17 35 646 211 100 [24] goat Capra hircus L 150 20 70 447 267 91 [25] pronghorn antelope Antilocapra americana 235 12 75 871 365 24 [26] black tail deer Odocoileus hemionus columbianus 150 15 200 -

Preliminary Analysis of European Small Mammal Faunas of the Eemian Interglacial: Species Composition and Species Diversity at a Regional Scale
Article Preliminary Analysis of European Small Mammal Faunas of the Eemian Interglacial: Species Composition and Species Diversity at a Regional Scale Anastasia Markova * and Andrey Puzachenko Institute of Geography, Russian Academy of Sciences, Staromonetny 29, Moscow 119017, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-495-959-0016 Academic Editors: Maria Rita Palombo and Valentí Rull Received: 22 May 2018; Accepted: 20 July 2018; Published: 26 July 2018 Abstract: Small mammal remains obtained from the European localities dated to the Eemian (Mikulino) age have been analyzed for the first time at a regional scale based on the present biogeographical regionalization of Europe. The regional faunas dated to the warm interval in the first part of the Late Pleistocene display notable differences in fauna composition, species richness, and diversity indices. The classification of regional faunal assemblages revealed distinctive features of small mammal faunas in Eastern and Western Europe during the Eemian (=Mikulino, =Ipswichian) Interglacial. Faunas of the Iberian Peninsula, Apennine Peninsula, and Sardinia Island appear to deviate from the other regions. In the Eemian Interglacial, the maximum species richness of small mammals (≥40 species) with a relatively high proportion of typical forest species was recorded in Western and Central Europe and in the western part of Eastern Europe. The lowest species richness (5–14 species) was typical of island faunas and of those in the north of Eastern Europe. The data obtained make it possible to reconstruct the distribution of forest biotopes and open habitats (forest-steppe and steppe) in various regions of Europe. Noteworthy is a limited area of forests in the south and in the northeastern part of Europe. -

Zeitschrift Für Säugetierkunde
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/ Z. Säugetierkunde 57 (1992) 364-372 © 1992 Verlag Paul Parey, Hamburg und Berlin ISSN 0044-3468 Capture-recapture study of a population of the Mediterranean Pine vole (Microtus duodecimcostatus) in Southern France By G. Guedon, E. Paradis, and H. Croset Laboratoire d'Eco-ethologie, Institut des Sciences de ['Evolution, Universite de Montpellier II, Montpellier, France Receipt of Ms. 28. 11. 1991 Acceptance of Ms. 3. 3. 1992 Abstract Investigated the population dynamics of a Microtus duodecimcostatus population by capture-recapture in Southern France during two years. The study was carried out in an apple orchard every three months on a 1 ha area. Numbers varied between 100 and 400 (minimum in summer). Reproduction occurred over the year and was lowest in winter. Renewal of the population occurred mainly in autumn. The population contained erratic individuals which did not take part in the reproduction. Resident individuals had a longer life-span and home ranges always located at the same place. Mean adult body weight varied only among females in relation to the reproductive rate. The observed demography of M. duodecimcostatus could be explained by biological traits (litter size, longevity) and by features of the habitat (high and constant level of resources, low level of disturbance), suggesting that social behaviours are an important regulating factor of numbers. Introduction The Mediterranean pine vole (Microtus duodecimcostatus de Selys-Longchamps) has a narrow geographic ränge: Portugal, Spain and Southern France (Niethammer 1982). Its population dynamics in natural habitats is unknown. The Mediterranean pine vole lives also in cultivated areas. -

Chlorophacinone Baiting for Belding's Ground Squirrels
CHLOROPHACINONE BAITING FOR BELDLNG'S GROUND SQUIRRELS CRAJG A. RAMEY, USDA, APHIS, Wildlife Services, National Wildlife Research Center, Fort Collins, CO, USA GEORGE H. MATSCHKE , USDA, APHIS, Wildlife Services, National Wildlife Research Center, Fort Collins, CO, USA RICHARD M. ENGEMAN, USDA, APHIS, Wildlife Services, National Wildlife Research Center, Fort Collins, CO, USA Abstract: The efficacy of using 0.0 l % chlorophacinone on steam-rolled oat (SRO) groats applied in CA alfalfa by spot-baiting /hand baiting around burrow entrances ( ~ l 1.5 g) to control free -ranging Belding's ground squirrels (Spermophilus beldingi) were compared in 6 randomly assigned square treatment units (TUs). Four TUs were given the rodenticide and 2 treated with placebo bait. Each TU was a 0.4 ha square surrounded by a similarly treated 5.5 ha square buffer zone. Baits were applied on May 13 and re-applied , on May 20 and May 22, after 7 days of un-forecasted cool wet weather greatly reduced their above ground activity. Pesticide (EPA SLN CA-890024) efficacy was calculated as% reduction (PR) of ground squirrels on each TUs measured directly by visual counts (VCs) and indirectly by active burrow counts (ABCs). VCs and ABCs provided mean PRs that met US EPA's 70% minimum standard efficacy threshold for field rodenticides (x = 73.5%, SD+ 13.3; x = 80%, SD ±_6.2, respectively). ANOVA results of the PRs were highly significant (F = 29.72, df 1/4, p = 0.0055 and F = 72.92, df l/4, P = 0.00 1, respectively) . All carcasses (38) located above ground were analyzed for pesticide and 80% had detectable levels in whole animals (x = 0.1131 ppm, SD ±_0.0928) . -

Spain and Portugal, 26 Dec 2019 – 9 Jan 2020. VLADIMIR DINETS
Spain and Portugal, 26 Dec 2019 – 9 Jan 2020. VLADIMIR DINETS Browsing trip reports on mammalwatching.com (my favorite way of self-torture for hardening the spirit), I noticed that there were none from mainland Portugal. So I talked my wife into going there for the winter break; we brought along my daughter and in-laws to make it more entertaining, so all mammalwatching was done at night or at dawn. We spent a few days in western Spain as well. Despite excellent weather I got only 31 species; goodies included Morisco roe deer, Pyrenean desman of the rarely seen western subspecies, Iberian shrew which I missed in 2014 despite much effort, and the recently split Portuguese field vole, the latter two seen within 40 min from each other. We forgot my camera and all flashlights at home, so I have no mammal photos except those taken with my phone. New moon was on December 26. Spain The weather was dry and cold in Spain, with strong temperature inversion in the interior (-2oC at night in Avila but +4 at 1700 m in Sierra de Gredos). Night drives and thermal scoping in fields, pastures and open woodland were remarkably devoid of mammal sightings. Avila: There are small colonies of Mediterranean pine voles in steeper portions of grassy slopes below the city walls, the best one at 40.659619N 4.704030W. Park across the street and watch the slope until you spot a vole; there is street lighting. A thermal scope helps. I saw one at 1 am after 2 hours of watching. -

Species-Specific Movement Traits and Specialization Determine the Spatial Responses of Small Mammals Towards Roads
Landscape and Urban Planning 169 (2018) 199–207 Contents lists available at ScienceDirect Landscape and Urban Planning journal homepage: www.elsevier.com/locate/landurbplan Research Paper Species-specific movement traits and specialization determine the spatial MARK responses of small mammals towards roads ⁎ Clara Griloa,b, , Guillem Molina-Vacasc, Xavier Fernández-Aguilarc, Juan Rodriguez-Ruizc, Victor Ramiroc, Flávia Porto-Peterc, Fernando Ascensãod,e, Jacinto Romána, Eloy Revillaa a Departamento de Biología de la Conservación, Estación Biológica de Doñana (EBD-CSIC), Calle Américo Vespucio s/n, E-41092 Sevilla, Spain b Setor Ecologia Departamento Biologia, Universidade Federal de Lavras, Campus Universitário, 37200-000, Lavras, Minas Gerais, Brazil c Universidade de Lisboa, Fundação da Faculdade de Ciências, C2 5°, 1749-016 Lisboa, Portugal d CIBIO/InBio, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus Agrário de Vairão, Vairão, Portugal e CEABN/InBio, Centro de Ecologia Aplicada “Professor Baeta Neves”, Instituto Superior de Agronomia, Universidade de Lisboa, Tapada da Ajuda, 1349-017 Lisboa, Portugal ARTICLE INFO ABSTRACT Keywords: The barrier effect is a pervasive impact of road networks. For many small mammals individual avoidance re- Avoidance sponses can be the mechanism behind the barrier effect. However, little attention has been paid to which species Barrier effect and road characteristics modulate road avoidance and mortality risk. We measured the strength of the barrier Highways effect imposed by the road on three rodent species with different body sizes and habitat specializations: Southern Mortality risk water vole (Arvicola sapidus), Mediterranean pine vole (Microtus duodecimcostatus) and Algerian mouse (Mus Rodents spretus). We analysed the effect of traffic intensity on use of space and direction of movement and the effect of Traffic volume road type (4-lane highway, 2-lane paved road and 1-lane unpaved road) on crossing rates with simulations of roads bisecting each home range. -
Canlilarin Siniflandirilmasi
CANLILARIN 16 SINIFLANDIRILMASI SINIFLANDIRMA Bugün, yaklaşık 1.6 milyon farklı organizma çeşidinin var olduğu bilinmekte ve her yıl birkaç bini daha teşhis edilmektedir. Bazı uzmanlar, 10 milyon kadar farklı organizma türünün var olduğunu kabul etmektedir. Organizmaların vücudu, 5 mikron çapındaki bakteriden 100 metreden daha boylu sekoya ağaçlarına değişmektedir. Bu çok büyük çeşitlilikteki organizma sayısı ile uğraşmak için, biyologlar, uluslararası geçerli bir sisteme göre, organizmaları tanılar ve adlandırırlar. Bu, çeşitli canlılar ve bunların özellikleri hakkında birbiriyle iletişim kuran bilim adamlarının işini kolaylaştırır. Taksonomi, canlıların sınıflandırılması ve adlandırılması ile ilgili biyoloji dalıdır. 16-1 Eski Sınıflandırma Tasarları Eski sınıflandırma girişimlerinin hepsinde, canlılar, bitkiler alemi ve hayvanlar alemi olarak iki büyük gruba ayrılmıştır. Bu iki grup da çeşitli şekillerde alt bölümlere ayrılmıştır. Sınıflandırmada ilk büyük ilerleme, İngiliz doğa bilimci John Ray tarafından 1600 'lerin ortasında yapılmıştır. Ray, 18.000 'den fazla farklı bitki çeşidini tanıladı ve sınıflandırdı. Aynı zamanda, birkaç değişik hayvan grubunun üyelerini de sınıflandırdı. Her bir farklı organizma çeşidi için tür terimini de ilk kullanan Ray olmuştur. Ray, bir türü, yapısal olarak aynı olan ve karakteristiklerini döllerine aktaran bir organizma grubu olarak tanımladı. Daha geniş bir grupta bir araya getirilen yakın akraba türlere cins adı verildi. Akraba cinsler bir sonra gelen daha geniş gruplara sıralandılar. İsveçli botanikçi Carolus Linnaeus çoğunlukla çağdaş taksonominin kurucusu olarak bilinir. Linnaeus, halen kullanılan, organizmaları sınıflandırma ve isimlendirme yöntemlerini kurmuştur. Fazlasıyla kullanışlı sisteminde, bitki ve hayvanlar kolaylıkla tanınabilecek bir şekilde düzenlenmiştir. Ray gibi, Linnaeus de, kurduğu sınıflandırma sisteminde, temel olarak yapısal benzerlikleri kullanmıştır. 16-2 Sınıflandırma Kategorileri Linnaeus ile günümüz arasındaki zamanda, taksonomistler sınıflandırma sistemine bazı kategoriler eklemişleredir. -
1 Energetics and Thermal Adaptation in Semifossorial Pine-Voles Microtus
Energetics and thermal adaptation in semifossorial pine-voles Microtus lusitanicus and Microtus duodecimcostatus Rita I. Monarca1*, John R. Speakman2,3 and Maria da Luz Mathias1 1 CESAM – Center for Environmental and Marine Studies, Departamento de Biologia Animal, Faculdade de Ciências da Universidade de Lisboa, Lisbon, Portugal 2 Institute of Biological and Environmental Sciences, University of Aberdeen, Tillydrone Ave, Aberdeen, Scotland, UK, 3 Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, 1 West Beichen Road, Chaoyang, Beijing, China *Corresponding author: Corresponding author: Rita I. Monarca CESAM – Center for Environmental and Marine Studies, Departamento de Biologia Animal, Faculdade de Ciências da Universidade de Lisboa, Lisboa, Portugal Tel: +351 217500000 ext 22303 Fax: +351 217500028 Email: [email protected] Keywords: Doubly labelled water; Resting metabolic rate; Water turnover, digging energetics 1 Abstract Rodents colonizing subterranean environments have developed several morphological, physiological and behaviour traits that promote the success of individuals in such demanding conditions. Resting metabolic rate, thermoregulation capacity and daily energy expenditure were analysed in two fossorial pine-vole species Microtus lusitanicus and M.duodecimcostatus inhabiting distinct areas of the Iberian Peninsula. Individuals were captured in locations with different habitat and soil features, allowing the comparison of energetic parameters with ecological characteristics, that can help explain the use of the subterranean environment and dependence of the burrow system. Results showed that M. duodecimcostatus has lower mass independent resting metabolic rate when compared with M. lusitanicus, which may be a response to environmental features of their habitat, such as dryer soils and lower water availability. Thermal conductance increased with body mass and was dependent on the ambient temperature.