1 Energetics and Thermal Adaptation in Semifossorial Pine-Voles Microtus
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Mammal Extinction Facilitated Biome Shift and Human Population Change During the Last Glacial Termination in East-Central Europeenikő
Mammal Extinction Facilitated Biome Shift and Human Population Change During the Last Glacial Termination in East-Central EuropeEnikő Enikő Magyari ( [email protected] ) Eötvös Loránd University Mihály Gasparik Hungarian Natural History Museum István Major Hungarian Academy of Science György Lengyel University of Miskolc Ilona Pál Hungarian Academy of Science Attila Virág MTA-MTM-ELTE Research Group for Palaeontology János Korponai University of Public Service Zoltán Szabó Eötvös Loránd University Piroska Pazonyi MTA-MTM-ELTE Research Group for Palaeontology Research Article Keywords: megafauna, extinction, vegetation dynamics, biome, climate change, biodiversity change, Epigravettian, late glacial Posted Date: August 11th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-778658/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/27 Abstract Studying local extinction times, associated environmental and human population changes during the last glacial termination provides insights into the causes of mega- and microfauna extinctions. In East-Central (EC) Europe, Palaeolithic human groups were present throughout the last glacial maximum (LGM), but disappeared suddenly around 15 200 cal yr BP. In this study we use radiocarbon dated cave sediment proles and a large set of direct AMS 14C dates on mammal bones to determine local extinction times that are compared with the Epigravettian population decline, quantitative climate models, pollen and plant macrofossil inferred climate and biome reconstructions and coprophilous fungi derived total megafauna change for EC Europe. Our results suggest that the population size of large herbivores decreased in the area after 17 700 cal yr BP, when temperate tree abundance and warm continental steppe cover both increased in the lowlands Boreal forest expansion took place around 16 200 cal yr BP. -

Microtus Duodecimcostatus) in Southern France G
Capture-recapture study of a population of the Mediterranean Pine vole (Microtus duodecimcostatus) in Southern France G. Guédon, E. Paradis, H Croset To cite this version: G. Guédon, E. Paradis, H Croset. Capture-recapture study of a population of the Mediterranean Pine vole (Microtus duodecimcostatus) in Southern France. Mammalian Biology, Elsevier, 1992, 57 (6), pp.364-372. ird-02061421 HAL Id: ird-02061421 https://hal.ird.fr/ird-02061421 Submitted on 8 Mar 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Capture-recapture study of a population of the Mediterranean Pine vole (Microtus duodecimcostatus) in Southern France By G. GUEDON, E. PARADIS, and H. CROSET Laboratoire d'Eco-éthologie, Institut des Sciences de l'Evolution, Université de Montpellier II, Montpellier, France Abstract Investigated the population dynamics of a Microtus duodecimcostatus population by capture- recapture in Southern France during two years. The study was carried out in an apple orchard every three months on an 1 ha area. Numbers varied between 100 and 400 (minimum in summer). Reproduction occurred over the year and was lowest in winter. Renewal of the population occurred mainly in autumn. -

Des Mammifères Sauvages D'aquitaine
Atlas des Mammifères sauvages d’Aquitaine Première synthèse sur les Mammifères d’Aquitaine, cet atlas est une base de connaissance des espèces de la région. Dotée d’entités biogéographiques variées, l’Aquitaine offre une diversité mammalogique d’une grande richesse qui mérite d’être prise en compte dans les politiques environnementales. L’atlas des Mammifères sauvages d’Aquitaine, composé de plusieurs tomes, décrypte la répartition de chaque espèce dans la région et Tome 6 : Les Rongeurs, les Erinacéomorphes et les Soricomorphes les Soricomorphes et les Erinacéomorphes 6 : Les Rongeurs, Tome fournit des éléments de compréhension sur l’état des populations. C’est un travail collectif et collaboratif entre de multiples partenaires qu’ils soient professionnels ou amateurs. Ce sixième et dernier ouvrage présente trois groupes d’espèces souvent peu - d’Aquitaine étudiées pour certaines d’entre elles : Les Rongeurs, les Erinacéomorphes et les Soricomorphes. Avec la participation d’un grand nombre de partenaires, le tome 6 de l’Atlas des Mammifères sauvages d’Aquitaine aborde 29 espèces présentes dans la région sous forme de monographies et de cartes de répartition. Le Campagnol souterrain ne dispose que d’une donnée confirmée et n’apparaît donc pas sous forme de monographie. Le Castor d’Eurasie, bientôt de retour, bénéficie lui d’une monographie. D’autres éléments en particulier sur les techniques d’inventaires ou les risques sanitaires viennent compléter ces monographies. des Mammifères sauvages sauvages des Mammifères Atlas des Mammifères -

The Evolutionary History and Conservation of an Endemic and Threatened Iberian Rodent: the Cabrera Vole (Microtus Cabrerae)
The evolutionary history and conservation of an endemic and threatened Iberian rodent: the Cabrera vole (Microtus cabrerae) D Soraia Isabel Moreira Barbosa Doutoramento em Biodiversidade, Genética e Evolução Departamento de Biologia 2017 Orientador Paulo Célio Alves, PhD, Professor Associado CIBIO/InBIO, Laboratório Associado, Universidade do Porto Coorientador Jeremy B Searle, PhD, Professor Department of Ecology and Evolutionary Biology, Cornell University FCUP iii The evolutionary history and conservation of an endemic and threatened Iberian rodent: the Cabrera vole (Microtus cabrerae) “…the study of life becomes a hollow, rarefied pursuit if the very animals and plants that fired our imaginations as children and triggered our curiosity as students should perish.” Michael E. Soulé and Bruce A. Wilcox FCUP v The evolutionary history and conservation of an endemic and threatened Iberian rodent: the Cabrera vole (Microtus cabrerae) Foreword In compliance with the no. 2 of article 4 of the General Regulation of Third Cycles of the University of Porto and with article 31 of the Decree-Law no. 74/2006, of 24 March, with the alteration introduced by the Decree-Law no. 230/2009, of 14 September, the results of already published works were totally used and included in some of the chapters of this dissertation. As there works were performed in collaboration with other authors, the candidate clarifies that, in all these works, participated in obtaining, interpreting, analysing and discussing the results, as well as in the writing of the published forms. This research was supported by Programa Operacional Potencial Humano – Quadro de Referência Estratégico National funds from the European Social fund and the Portuguese Ministério de Educação e Ciência through a PhD fellowship (Fundação para a Ciência e a Tecnologia, FCT - SFRH/BD/77726/2011). -

Table of Contents
Table of Contents Table of Contents Welcome Note of the German Research Platform for Zoonoses ........................................................ 2 Welcome Note of the Federal Government ...................................................................................... 3 Program Zoonoses 2019 - International Symposium on Zoonoses Research ...................................... 5 General information ...................................................................................................................... 14 Floor Plan .................................................................................................................................... 18 About the German Research Platform for Zoonoses ........................................................................ 19 Oral Presentations ........................................................................................................................ 20 Plenary Session I: Keynotes .......................................................................................................... 21 Session 1: Innate and Adaptive Immune Response......................................................................... 24 Session 2: Public Health ................................................................................................................ 31 Session 3: New and Re-Emerging Zoonotic Diseases ...................................................................... 38 Session 4: Diagnostics and NGS ................................................................................................... -

What Does the Oxygen Isotope Composition of Rodent Teeth Record?
Earth and Planetary Science Letters 361 (2013) 258–271 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl What does the oxygen isotope composition of rodent teeth record? Aure´lien Royer a,e, Christophe Le´cuyer a,f,n, Sophie Montuire b,e, Romain Amiot a, Serge Legendre a, Gloria Cuenca-Besco´ s c, Marcel Jeannet d, Franc-ois Martineau a a Laboratoire de Ge´ologie de Lyon, UMR CNRS 5276, Universite´ Lyon 1 et Ecole Normale Supe´rieure de Lyon, 69622 Villeurbanne, France b Bioge´osciences, UMR CNRS 5561, Universite´ de Bourgogne, 6 Boulevard Gabriel, 21000 Dijon, France c Departamento de Ciencias de la Tierra, A´rea de Paleontologı´a, Edificio de Geolo´gicas, 50009 Zaragoza, Spain d LAMPEA, UMR 6636, MMSH, 5 rue du Chateauˆ de l’Horloge, BP 647, 13094 Aix-en-Provence Cedex 2, France e Laboratoire Pale´obiodiversite´ et Evolution, EPHE-Ecole Pratique des Hautes Etudes, 21000 Dijon, France f Institut Universitaire de France, Paris, France article info abstract 18 Article history: Oxygen isotope compositions of tooth phosphate (d Op) were measured in 107 samples defined on the Received 6 December 2011 basis of teeth obtained from 375 specimens of extant rodents. These rodents were sampled from pellets Received in revised form collected in Europe from 381N (Portugal) to 651N (Finland) with most samples coming from sites 25 September 2012 18 located in France and Spain. Large oxygen isotopic variability in d Op is observed both at the intra- and Accepted 28 September 2012 inter-species scale within pellets from a given location. -

Hystrx It. J. Mamm. (Ns) Supp. (2007) V European Congress of Mammalogy
Hystrx It. J. Mamm . (n.s.) Supp. (2007) V European Congress of Mammalogy RODENTS AND LAGOMORPHS 51 Hystrx It. J. Mamm . (n.s.) Supp. (2007) V European Congress of Mammalogy 52 Hystrx It. J. Mamm . (n.s.) Supp. (2007) V European Congress of Mammalogy A COMPARATIVE GEOMETRIC MORPHOMETRIC ANALYSIS OF NON-GEOGRAPHIC VARIATION IN TWO SPECIES OF MURID RODENTS, AETHOMYS INEPTUS FROM SOUTH AFRICA AND ARVICANTHIS NILOTICUS FROM SUDAN EITIMAD H. ABDEL-RAHMAN 1, CHRISTIAN T. CHIMIMBA, PETER J. TAYLOR, GIANCARLO CONTRAFATTO, JENNIFER M. LAMB 1 Sudan Natural History Museum, Faculty of Science, University of Khartoum P. O. Box 321 Khartoum, Sudan Non-geographic morphometric variation particularly at the level of sexual dimorphism and age variation has been extensively documented in many organisms including rodents, and is useful for establishing whether to analyse sexes separately or together and for selecting adult specimens to consider for subsequent data recording and analysis. However, such studies have largely been based on linear measurement-based traditional morphometric analyses that mainly focus on the partitioning of overall size- rather than shape-related morphological variation. Nevertheless, recent advances in unit-free, landmark/outline-based geometric morphometric analyses offer a new tool to assess shape-related morphological variation. In the present study, we used geometric morphometric analysis to comparatively evaluate non-geographic variation in two geographically disparate murid rodent species, Aethmoys ineptus from South Africa and Arvicanthis niloticus from Sudan , the results of which are also compared with previously published results based on traditional morphometric data. Our results show that while the results of the traditional morphometric analyses of both species were congruent, they were not sensitive enough to detect some signals of non-geographic morphological variation. -

Swimming Ability in Three Costa Rican Dry Forest Rodents
Rev. Biol. Trop. 49(3-4): 1177-1181, 2001 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Swimming ability in three Costa Rican dry forest rodents William M. Cook1, Robert M. Timm1 and Dena E. Hyman2 1 Department of Ecology and Evolutionary Biology & Natural History Museum, Dyche Hall, University of Kansas, Lawrence, KS 66045 USA. Fax: (785) 864-5335. E-mail: [email protected]. 2 Department of Biology, University of Miami, Coral Gables, FL 33124 USA. Received 18-VII-2000. Corrected 20-III-2001. Accepted 30-III-2001. Abstract: We investigated the swimming abilities of three Costa Rican dry forest rodents (Coues’ rice rat, Oryzomys couesi, hispid cotton rat, Sigmodon hispidus, and spiny pocket mouse, Liomys salvini) associated with a large marsh, Laguna Palo Verde, using 90 s swim trials in a plastic container. Swimming ability was evaluated by observing the use of limbs and tail in the water, inclination to the surface, and diving and float- ing behavior. Rice rats could float, swim and dive, suggesting that they can exploit surface and underwater resources. Cotton rats swam at the water’s surface, but were less skilled swimmers than rice rats. Spiny pocket mice tired quickly and had difficulty staying at the water’s surface. Results suggest that differential swimming ability is related to the distribution of the three sympatric species within the marsh and adjacent forest habitats. Key words: Dry forest, Liomys salvini, Oryzomys couesi, Sigmodon hispidus, swimming ability. Most rodents can swim when necessary, level, which in recent years has been domina- and may even dive and swim skillfully (Dagg ted by dense stands of emergent cattails and Windsor 1972). -
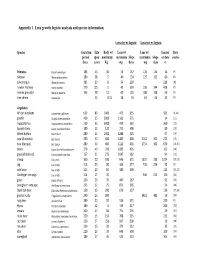
Appendix 1. Lens Growth Logistic Analysis and Species Information
Appendix 1. Lens growth logistic analysis and species information. Lens dry wt logistic Lens wet wt logistic Species Gestation Life Body wt Lens wt Lens wt Lenses Data period span maximum maximum Slope maximum Slope or data source days years Kg mg days mg days n Primates Papio hamadryas 183 45 30 55 167 178 124 16 PS baboon Macaca fascicularis 180 38 5 40 130 125 162 60 PS cynomolgus Alouatta caraya 185 20 8 34 220 228 [8] howler monkey Homo sapiens 270 115 5 45 359 198 244 608 PS human prenatal Macaca mulatta 165 38 12 60 125 188 108 50 PS tree shrew Tupaia glis 12 0.15 28 76 63 63 32 PS Ungulates African elephant Loxodonta a africana 659 80 5000 475 935 561 [9,10] giraffe Giraffa camelopardalis 460 35 2000 1163 571 34 [11] hipppotamus Hippopotamus amphibius 250 55 3000 410 362 360 [12] Spanish Ibex Capra pyrenaica Schinz 180 12 120 711 508 80 [13] Wood buffalo Bison bison 280 25 1000 1288 525 97 [14] cow (Australia) Bos taurus 280 30 600 1483 400 3162 302 231 [15] cow (Europe) Bos taurus 280 30 400 1122 401 2754 302 630 [16,17] zebra Equus burchelli antiquorum 370 40 350 1085 436 102 [18] gnu (wildebeest) Connochaetes taurinus 257 21 275 1047 362 94 [19] sheep Ovis aries 160 20 100 646 311 1622 239 1224 [20,21] pig Sus scrofa 115 25 80 364 277 736 254 92 PS wild boar Sus scrofa 115 20 84 389 309 113 [22,23] Gottingen minipigs Sus scrofa 114 17 35 646 211 100 [24] goat Capra hircus L 150 20 70 447 267 91 [25] pronghorn antelope Antilocapra americana 235 12 75 871 365 24 [26] black tail deer Odocoileus hemionus columbianus 150 15 200 -

Comparative Phylogeography As an Integrative Approach to Understand Human and Other Mammal
Comparative phylogeography as an integrative approach to understand human and other mammal distributions in Europe Luis Oxala García Rodríguez A thesis submitted in partial fulfilment of the requirements of Bournemouth University for the degree of Doctor of Philosophy June 2019 This page intentionally left blank II This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and due acknowledgement must always be made of the use of any material contained in, or derived from, this thesis. III Comparative phylogeography as an integrative approach to understand human and other mammal distributions in Europe. Oxala García Rodríguez Abstract Phylogeography refers to the phylogenetic analysis of organisms in the context of their geographical distribution. The analytical methods build phylogenetic trees and networks from haplotypes in order to investigate the history of the organisms. Phylogeographic studies have revealed the importance of climatic oscillations and the role of the Last Glacial Maximum (27,500 to 16,000 years ago) with the formation of refugia where distinct haplotypes originate in Europe. The population expansions and contractions into these refugial areas have driven the evolution of different lineages but the similarities and differences between species are still poorly understood. This thesis aims to gain a better understanding of the phylogeographical processes of different mammals’ species in Europe. This was done by collecting published mitochondrial DNA control region sequences of 29 different species and analysing them individually and comparatively. This research presents a standardised way of understanding phylogeography from the mitochondrial DNA perspective to improve the comparison of studies in the field. -

Preliminary Analysis of European Small Mammal Faunas of the Eemian Interglacial: Species Composition and Species Diversity at a Regional Scale
Article Preliminary Analysis of European Small Mammal Faunas of the Eemian Interglacial: Species Composition and Species Diversity at a Regional Scale Anastasia Markova * and Andrey Puzachenko Institute of Geography, Russian Academy of Sciences, Staromonetny 29, Moscow 119017, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-495-959-0016 Academic Editors: Maria Rita Palombo and Valentí Rull Received: 22 May 2018; Accepted: 20 July 2018; Published: 26 July 2018 Abstract: Small mammal remains obtained from the European localities dated to the Eemian (Mikulino) age have been analyzed for the first time at a regional scale based on the present biogeographical regionalization of Europe. The regional faunas dated to the warm interval in the first part of the Late Pleistocene display notable differences in fauna composition, species richness, and diversity indices. The classification of regional faunal assemblages revealed distinctive features of small mammal faunas in Eastern and Western Europe during the Eemian (=Mikulino, =Ipswichian) Interglacial. Faunas of the Iberian Peninsula, Apennine Peninsula, and Sardinia Island appear to deviate from the other regions. In the Eemian Interglacial, the maximum species richness of small mammals (≥40 species) with a relatively high proportion of typical forest species was recorded in Western and Central Europe and in the western part of Eastern Europe. The lowest species richness (5–14 species) was typical of island faunas and of those in the north of Eastern Europe. The data obtained make it possible to reconstruct the distribution of forest biotopes and open habitats (forest-steppe and steppe) in various regions of Europe. Noteworthy is a limited area of forests in the south and in the northeastern part of Europe. -

Arvicola Sapidus ) En Un Ambient Ripari De Muntanya Mediterrània
Ecologia espaciotemporal i relacions intra - i interespecífiques de la rata d'aigua ( Arvicola sapidus ) en un ambient ripari de muntanya mediterrània Isabe l Mate i Alonso A questa tesi doctoral està subjecta a la llicència Reconeixement - NoComercial 3.0. Espanya de Creative Commons . Esta tesis doctoral está sujeta a la licencia Reconocimiento - NoComercial 3.0. España de Creati ve Commons . Th is doctoral thesis is licensed under the Creative Commons Attribution - NonCommercia l 3.0. Spain License . Ecologia espaciotemporal i relacions intra‐ i interespecífiques de la rata d'aigua (Arvicola sapidus) en un ambient ripari de muntanya mediterrània Isabel Mate i Alonso Facultat de Biologia Departament de Biologia Animal Programa de Doctorat en Biodiversitat Ecologia espaciotemporal i relacions intra‐ i interespecífiques de la rata d'aigua (Arvicola sapidus) en un ambient ripari de muntanya mediterrània Memòria presentada per Isabel Mate i Alonso per a optar al títol de Doctora per la Universitat de Barcelona Barcelona, octubre 2015 Isabel Mate i Alonso (Doctoranda) Dr.Joaquim Gosàlbez Noguera Dr. Jordi Ruiz Olmo Dr. Miquel Salicrú Pagès (Director/tutor) (Director) (Director) Dep. Biologia Animal, Dep. Agricultura, Dep. d’Estadística, Facultat de Biologia, Ramaderia, Pesca, Facultat de Biologia Universitat de Barcelona Alimentació i Medi Natural Universitat de Barcelona Generalitat de Catalunya Quan surts per fer el viatge cap a Ítaca, has de pregar que el camí sigui llarg, ple d'aventures, ple de coneixences. Has de pregar que el camí sigui llarg, que siguin moltes les matinades que entraràs en un port que els teus ulls ignoraven, i vagis a ciutats per aprendre dels que saben.