Mitochondrial Dynamics in Parasitic Protists
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Planktonic Protist Interactome: Where Do We Stand After a Century of Research?
bioRxiv preprint doi: https://doi.org/10.1101/587352; this version posted May 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Bjorbækmo et al., 23.03.2019 – preprint copy - BioRxiv The planktonic protist interactome: where do we stand after a century of research? Marit F. Markussen Bjorbækmo1*, Andreas Evenstad1* and Line Lieblein Røsæg1*, Anders K. Krabberød1**, and Ramiro Logares2,1** 1 University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N- 0316 Oslo, Norway 2 Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta, 37-49, ES-08003, Barcelona, Catalonia, Spain * The three authors contributed equally ** Corresponding authors: Ramiro Logares: Institute of Marine Sciences (ICM-CSIC), Passeig Marítim de la Barceloneta 37-49, 08003, Barcelona, Catalonia, Spain. Phone: 34-93-2309500; Fax: 34-93-2309555. [email protected] Anders K. Krabberød: University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N-0316 Oslo, Norway. Phone +47 22845986, Fax: +47 22854726. [email protected] Abstract Microbial interactions are crucial for Earth ecosystem function, yet our knowledge about them is limited and has so far mainly existed as scattered records. Here, we have surveyed the literature involving planktonic protist interactions and gathered the information in a manually curated Protist Interaction DAtabase (PIDA). In total, we have registered ~2,500 ecological interactions from ~500 publications, spanning the last 150 years. -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

Predatory Flagellates – the New Recently Discovered Deep Branches of the Eukaryotic Tree and Their Evolutionary and Ecological Significance
Protistology 14 (1), 15–22 (2020) Protistology Predatory flagellates – the new recently discovered deep branches of the eukaryotic tree and their evolutionary and ecological significance Denis V. Tikhonenkov Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok, 152742, Russia | Submitted March 20, 2020 | Accepted April 6, 2020 | Summary Predatory protists are poorly studied, although they are often representing important deep-branching evolutionary lineages and new eukaryotic supergroups. This short review/opinion paper is inspired by the recent discoveries of various predatory flagellates, which form sister groups of the giant eukaryotic clusters on phylogenetic trees, and illustrate an ancestral state of one or another supergroup of eukaryotes. Here we discuss their evolutionary and ecological relevance and show that the study of such protists may be essential in addressing previously puzzling evolutionary problems, such as the origin of multicellular animals, the plastid spread trajectory, origins of photosynthesis and parasitism, evolution of mitochondrial genomes. Key words: evolution of eukaryotes, heterotrophic flagellates, mitochondrial genome, origin of animals, photosynthesis, predatory protists, tree of life Predatory flagellates and diversity of eu- of the hidden diversity of protists (Moon-van der karyotes Staay et al., 2000; López-García et al., 2001; Edg- comb et al., 2002; Massana et al., 2004; Richards The well-studied multicellular animals, plants and Bass, 2005; Tarbe et al., 2011; de Vargas et al., and fungi immediately come to mind when we hear 2015). In particular, several prevailing and very abun- the term “eukaryotes”. However, these groups of dant ribogroups such as MALV, MAST, MAOP, organisms represent a minority in the real diversity MAFO (marine alveolates, stramenopiles, opistho- of evolutionary lineages of eukaryotes. -
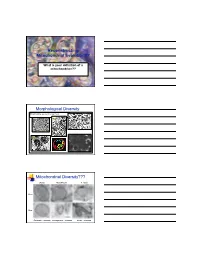
Reconstructing Mitochondrial Evolution?? Morphological Diversity Mitochondrial Diversity???
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial Diversity??? Chicken Neocallimastix T. foetus 100 nm 50 nm Entamoeba - mitosome microsporidian - mitosome Giardia - mitosome Reconstructing Evolution Mitochondrial evolution well established endosymbiotic theory α-proteobacterium - Rickettsia prowazekii Hydrogenosomal evolution No DNA NOW 2 examples Nyctotherus and Blastocystis (MLO) Several proteins similar to mitochondria Mitosome evolution No DNA Few proteins identified similar to mitochondria Origins via Endosymbiosis Current dogma - mitochondria and related Aerobic α-proteobacterium prokaryote gave rise to present day mitochondria. organelles arose just once in evolution Are hydrogenosomes and mitosomes of anaerobic protists derived from the same proto-mitochondrion? Evidence for: accumulating evidence for several proteins that are currently found in mitochondria - Proteins of Fe-S cluster formation. Scenario A Common ancestor facultative Scenario B organism? degenerate mitochondrion invoke lateral gene transfer from anaerobic prokaryotes Hypotheses for Mito Acquisition Mitochondrion-related Organelles 2 way to interpret this summary - can you think of both? Groups with mitochondrial homologues Hjort et al Phil. Trans. R. Soc. B 2010 365, 713-727 Origin of Hydrogenosomes (Mitosomes?) 1. a)Conversion of Mitochondria HYD b)Common ancestor with mitochondria ? HYD 2. Independent Origin α-proteobacterium ? - anaerobic bacterium HYD Organelles - origins and biogenesis Approaches: (1) Conduct phylogenetic analyses of similar proteins Hsp70 Fd Hsp60 Isc subunits (2) Examine protein targeting to the organelle matrix protein targeting membrane protein targeting (3) Characterize membrane/translocation components These components could have evolved as the endosymbiont was converted to organelle. Reveals evolutionary history. -

A KEY to the COMMON PARASITIC PROTOZOANS of NORTH AMERICAN FISHES Thomas L. Wellborn, Jr. and Wilmer A. Rogers Zoology-Ent
. A KEY to the COMMON PARASITIC PROTOZOANS of NORTH AMERICAN FISHES Thomas L. Wellborn, Jr. and Wilmer A. Rogers Zoology-Entomology Department Series Fisheries No. 4 AGRICULTURAL EXPERIMENT STATION AUBURN UNIVERSITY E. V. Smith, Director March 1966 Auburn, Alabama (Revised June 1970) A KEY TO THE COMMON PARASITIC PROTOZOANS 1 OF NORTH AMERICAN FISHES Thomas L. Wellborn, Jr. 2/— and Wilmer A. Rogers 3/— Private, state, and federal fish husbandry industries suffer great losses each year because of disease and parasites. The parasitic protozoans included in this key are the ones most commonly associated with fish mortalities. A total of 23 genera of parasitic protozoans may be identified by use of this key. The fish protozoan parasites are responsible for a large part of the mortalities that occur at fish hatcheries each year. This is because they are capable of building up tremendous populations within relatively short periods of time, and some are capable of causing extreme damage to fish. Proper treatment and control of the diseases caused by the various protozoans are impossible without knowing their identity. This key will be helpful to fishery workers in identifying the more common genera. It must be remembered, however, that a microscope and knowledge of its use are absolute prerequisites for identifying protozoans. Certain parasitic protozoans cannot be identified below the rank of Order - without use of special techniques; therefore, all known genera are not included in the herein reported key. Protozoans belonging to such Orders should be sent to a specialist for identification. 1/ Supported in part by Southeastern Cooperative Fish Parasite and Disease Project (Fish Restoration Funds). -

Novel Lineages of Oxymonad Flagellates from the Termite Porotermes Adamsoni (Stolotermitidae): the Genera Oxynympha and Termitim
Protist, Vol. 170, 125683, December 2019 http://www.elsevier.de/protis Published online date 21 October 2019 ORIGINAL PAPER Novel Lineages of Oxymonad Flagellates from the Termite Porotermes adamsoni (Stolotermitidae): the Genera Oxynympha and Termitimonas a,1 b a c b,1 Renate Radek , Katja Meuser , Samet Altinay , Nathan Lo , and Andreas Brune a Evolutionary Biology, Institute for Biology/Zoology, Freie Universität Berlin, 14195 Berlin, Germany b Research Group Insect Gut Microbiology and Symbiosis, Max Planck Institute for Terrestrial Microbiology, 35043 Marburg, Germany c School of Life and Environmental Sciences, The University of Sydney, Sydney, NSW 2006, Australia Submitted January 21, 2019; Accepted October 9, 2019 Monitoring Editor: Alastair Simpson The symbiotic gut flagellates of lower termites form host-specific consortia composed of Parabasalia and Oxymonadida. The analysis of their coevolution with termites is hampered by a lack of informa- tion, particularly on the flagellates colonizing the basal host lineages. To date, there are no reports on the presence of oxymonads in termites of the family Stolotermitidae. We discovered three novel, deep-branching lineages of oxymonads in a member of this family, the damp-wood termite Porotermes adamsoni. One tiny species (6–10 m), Termitimonas travisi, morphologically resembles members of the genus Monocercomonoides, but its SSU rRNA genes are highly dissimilar to recently published sequences of Polymastigidae from cockroaches and vertebrates. A second small species (9–13 m), Oxynympha loricata, has a slight phylogenetic affinity to members of the Saccinobaculidae, which are found exclusively in wood-feeding cockroaches of the genus Cryptocercus, the closest relatives of termites, but shows a combination of morphological features that is unprecedented in any oxymonad family. -

Ectocarpus: an Evo‑Devo Model for the Brown Algae Susana M
Coelho et al. EvoDevo (2020) 11:19 https://doi.org/10.1186/s13227-020-00164-9 EvoDevo REVIEW Open Access Ectocarpus: an evo-devo model for the brown algae Susana M. Coelho1* , Akira F. Peters2, Dieter Müller3 and J. Mark Cock1 Abstract Ectocarpus is a genus of flamentous, marine brown algae. Brown algae belong to the stramenopiles, a large super- group of organisms that are only distantly related to animals, land plants and fungi. Brown algae are also one of only a small number of eukaryotic lineages that have evolved complex multicellularity. For many years, little information was available concerning the molecular mechanisms underlying multicellular development in the brown algae, but this situation has changed with the emergence of Ectocarpus as a model brown alga. Here we summarise some of the main questions that are being addressed and areas of study using Ectocarpus as a model organism and discuss how the genomic information, genetic tools and molecular approaches available for this organism are being employed to explore developmental questions in an evolutionary context. Keywords: Ectocarpus, Life-cycle, Sex determination, Gametophyte, Sporophyte, Brown algae, Marine, Complex multicellularity, Phaeoviruses Natural habitat and life cycle Ectocarpus is a cosmopolitan genus, occurring world- Ectocarpus is a genus of small, flamentous, multicellu- wide in temperate and subtropical regions, and has been lar, marine brown algae within the order Ectocarpales. collected on all continents except Antarctica [1]. It is pre- Brown algae belong to the stramenopiles (or Heter- sent mainly on rocky shores where it grows on abiotic okonta) (Fig. 1a), a large eukaryotic supergroup that (rocks, pebbles, dead shells) and biotic (other algae, sea- is only distantly related to animals, plants and fungi. -

P1891 Trichomonas Vaginalis Lifecycle Revisited
P1891 Trichomonas vaginalis lifecycle revisited Piotr Kochan1, Agata Pietrzyk2, Alban Vogel*3, Maria Gołębiowska3, Karan Kumar3, Eivind Krågebakk3, Andreas Talgø Lie3, Rebekka Nilsen3, Vilde Strøm3, Michael Zbiegien3, Barbara Papir4, Małgorzata Bulanda5 1Chair of Microbiology, Department of Bacteriology, Microbial Ecology and Parasitology, Kraków, Poland, 2Chair of Microbiology, Head of Parasitology Laboratory, Kraków, , 3School of Medicine in English, Jagiellonian University, Parasitology Research Circle, Kraków, Poland, 4Chair of Microbiology , Parasitology Laboratory, Kraków, , 5Jagiellonian University Medical College, Head of the Chair of Microbiology, Kraków, Poland Background: The World Health Organization (WHO) states there are 1 million new sexually transmitted infections (STI) occurring daily. Among these, Trichomonas vaginalis with over 140 million infected is ranked 4th, just after HBV with over 250 million infected, HPV with over 290 million and HSV ranked 1st with over 500 million infected. Despite the availability of treatment of this protozoan, it’s still prevalent both in the developing and developed countries, although chlamydial infections are on the rise, currently ranked as 5th by WHO. Trichomoniasis is twice as common as gonorrhea (~78 million), almost 4 times more common than HIV infections (~37 million) and 25 times more common than syphilis (~6 million). Trichomonas infection not only facilitates HIV acquisition due to epithelial damage, but also promotes HIV propagation. We aimed to re-evaluate our current knowledge on the lifecycle of Trichomonas vaginalis, including not only its multiplication, but also possible genetic exchange. Materials/methods: The study made use of two strains of Trichomonas vaginalis: reference strain ATCC-PRA-92 and the strain from the Chair of Microbiology of Jagiellonian University Medical College. -

23.3 Groups of Protists
Chapter 23 | Protists 639 cysts that are a protective, resting stage. Depending on habitat of the species, the cysts may be particularly resistant to temperature extremes, desiccation, or low pH. This strategy allows certain protists to “wait out” stressors until their environment becomes more favorable for survival or until they are carried (such as by wind, water, or transport on a larger organism) to a different environment, because cysts exhibit virtually no cellular metabolism. Protist life cycles range from simple to extremely elaborate. Certain parasitic protists have complicated life cycles and must infect different host species at different developmental stages to complete their life cycle. Some protists are unicellular in the haploid form and multicellular in the diploid form, a strategy employed by animals. Other protists have multicellular stages in both haploid and diploid forms, a strategy called alternation of generations, analogous to that used by plants. Habitats Nearly all protists exist in some type of aquatic environment, including freshwater and marine environments, damp soil, and even snow. Several protist species are parasites that infect animals or plants. A few protist species live on dead organisms or their wastes, and contribute to their decay. 23.3 | Groups of Protists By the end of this section, you will be able to do the following: • Describe representative protist organisms from each of the six presently recognized supergroups of eukaryotes • Identify the evolutionary relationships of plants, animals, and fungi within the six presently recognized supergroups of eukaryotes • Identify defining features of protists in each of the six supergroups of eukaryotes. In the span of several decades, the Kingdom Protista has been disassembled because sequence analyses have revealed new genetic (and therefore evolutionary) relationships among these eukaryotes. -

That of a Typical Flagellate. the Flagella May Equally Well Be Called Cilia
ZOOLOGY; KOFOID AND SWEZY 9 FLAGELLATE AFFINITIES OF TRICHONYMPHA BY CHARLES ATWOOD KOFOID AND OLIVE SWEZY ZOOLOGICAL LABORATORY, UNIVERSITY OF CALIFORNIA Communicated by W. M. Wheeler, November 13, 1918 The methods of division among the Protozoa are of fundamental signifi- cance from an evolutionary standpoint. Unlike the Metazoa which present, as a whole, only minor variations in this process in the different taxonomic groups and in the many different types of cells in the body, the Protozoa have evolved many and widely diverse types of mitotic phenomena, which are Fharacteristic of the groups into which the phylum is divided. Some strik- ing confirmation of the value of this as a clue to relationships has been found in recent work along these lines. The genus Trichonympha has, since its discovery in 1877 by Leidy,1 been placed, on the one hand, in the ciliates and, on the other, in the flagellates, and of late in an intermediate position between these two classes, by different investigators. Certain points in its structure would seem to justify each of these assignments. A more critical study of its morphology and especially of its methods of division, however, definitely place it in the flagellates near the Polymastigina. At first glance Trichonympha would undoubtedly be called a ciliate. The body is covered for about two-thirds of its surface with a thick coating of cilia or flagella of varying lengths, which stream out behind the body. It also has a thick, highly differentiated ectoplasm which contains an alveolar layer as well as a complex system of myonemes. -

49Th Jírovec's Protozoological Days
49th Jírovec's Protozoological Days Conference Proceedings Faculty of Science Charles University Prague, Czech Republic 2019 th 49 Jírovec's Protozoological Days Conference Proceedings Faculty of Science, Charles University, 2019 FOREWORD Dear protistologists, colleagues, and friends! Welcome to the, nearly jubilee, 49th Jírovec‟s Protozoological Days, an international protistological conference, traditionally organized by the Protozoological section of the Czech Society for Parasitology. This year„s Protodays are organized by a group from The Faculty of Science, Charles University, where 90 years ago Otto Jírovec received his doctorate and set out the foundations of Czech protistology. Since 1969, the conference has been an opportunity for graduate and postgraduate students, as well as postdocs and young scientists to present their research and results to the scientific community and the international community of protistologists. The topics presented at the conference include areas such as protistology, cell and molecular biology, genomics, phylogeny and systematics, as well as parasitology, biochemistry, and evolutionary biology and include discoveries of significant new organisms, metabolic pathways of organisms important to our understanding of the origin and evolution of life, research on the causes of serious parasitic diseases such as malaria, sleeping sickness, leishmaniasis, and many others, the development of their treatment and prevention. Thanks to English as the main language at the conference and thanks to the growing interest -

Mitosomes in Entamoeba Histolytica Contain a Sulfate Activation Pathway
Mitosomes in Entamoeba histolytica contain a sulfate activation pathway Fumika Mi-ichi, Mohammad Abu Yousuf, Kumiko Nakada-Tsukui, and Tomoyoshi Nozaki1 Department of Parasitology, National Institute of Infectious Diseases, Tokyo 162-8640, Japan Edited by Andrew Roger, Dalhousie University, Halifax, NS, Canada, and accepted by the Editorial Board October 19, 2009 (received for review June 25, 2009) Hydrogenosomes and mitosomes are mitochondrion-related or- and M. balamuthi lack the ISC system, and instead possess the ganelles in anaerobic/microaerophilic eukaryotes with highly re- nitrogen fixation (NIF) system, which is most likely derived from an duced and divergent functions. The full diversity of their content ancestral nitrogen fixing -proteobacterium by lateral gene transfer and function, however, has not been fully determined. To under- (22, 24). Only 5 proteins have been demonstrated in E. histolytica stand the central role of mitosomes in Entamoeba histolytica,a mitosomes: Cpn60 (8–10, 12), Cpn10 (13), mitochondrial Hsp70 parasitic protozoon that causes amoebic dysentery and liver ab- (11, 15), pyridine nucleotide transhydrogenase (PNT) (2, 8), and scesses, we examined the proteomic profile of purified mitosomes. mitochondria carrier family (MCF, ADP/ATP transporter) (14), Using 2 discontinuous Percoll gradient centrifugation and MS and the central role of mitosomes in E. histolytica remains unknown. analysis, we identified 95 putative mitosomal proteins. Immuno- Analysis of the genome of E. histolytica has not revealed any fluorescence assay showed that 3 proteins involved in sulfate additional information regarding the function of mitosomes and activation, ATP sulfurylase, APS kinase, and inorganic pyrophos- thus, a proteomic analysis of mitosomes seems to be the best phatase, as well as sodium/sulfate symporter, involved in sulfate approach to understand its structure and function (1, 2).