Phase and Chemical Equilibria in Multicomponent Fluid Systems with a Chemical Reaction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

N-Propyl Acetate Technical Data Sheet
Technical Data Sheet Product Name n-Propyl Acetate Synonyms Acetic Acid, n-propyl Ester Chemical Formula CH3COOC3H7 Product Description N-propyl acetate is a colorless, volatile solvent with an odor similar to acetone. It has good solvency power for many natural and synthetic resins. It is miscible with many organic solvents. Applications • Coatings • Wood lacquers • Aerosol sprays • Nail care • Cosmetic / personal care solvent • Fragrance solvent • Process solvent • Printing inks (especially flexographic and special screen) Typical Physical Properties Property Value Molecular Weight (g/mol) 102.13 Boiling Point @ 760 mmHg, 1.01 ar 101.5 °C (214.7 °F) Flash Point (Setaflash Closed Cup) 11.8 °C (53.24°F) Freezing Point -93 °C (-135.4°F) Vapor pressure@ 25°C — extrapolated 25 mmHg 4.79 Kpa Specific gravity (20/20°C) 0.888 Liquid Density @ 20°C 0.89 g/cm3 Vapor Density (air = 1) 3.5 Viscosity (cP or mPa•s @ 20°C) 0.6 Surface tension (dynes/cm or mN/m @ 20°C) 24.4 Specific heat (J/g/°C @ 25°C) No test data available Heat of vaporization (J/g) at normal boiling No test data available point Net heat of combustion (kJ/g) — predicted @ No test data available 25°C Autoignition temperature 380 °C (716 °F) Evaporation rate (n-butyl acetate = 1.0) 2.75 Solubility, g/L or % @ 20°C Solvent in water 2% Water in solvent 2.6% Hansen solubility parameters (J/cm³)1/2 _Total 8.6 _Non-Polar 7.5 _Polar 2.1 Form No. 327-00024-0812 Page 1 of 3 ®™Trademark of The Dow Chemical Company (“Dow”) or an affiliated company of Dow _Hydrogen bonding 3.7 Partition Coefficient, n-octanol/water 1.4 (log Pow) Flammable limits (vol.% in air) Lower 1.7 Upper 8.0 Typical Physical Properties: This data provided for those properties are typical values, and should not be construed as sales specifications. -

Comprehensive Characterization of Toxicity of Fermentative Metabolites on Microbial Growth Brandon Wilbanks1 and Cong T
Wilbanks and Trinh Biotechnol Biofuels (2017) 10:262 DOI 10.1186/s13068-017-0952-4 Biotechnology for Biofuels RESEARCH Open Access Comprehensive characterization of toxicity of fermentative metabolites on microbial growth Brandon Wilbanks1 and Cong T. Trinh1,2* Abstract Background: Volatile carboxylic acids, alcohols, and esters are natural fermentative products, typically derived from anaerobic digestion. These metabolites have important functional roles to regulate cellular metabolisms and broad use as food supplements, favors and fragrances, solvents, and fuels. Comprehensive characterization of toxic efects of these metabolites on microbial growth under similar conditions is very limited. Results: We characterized a comprehensive list of thirty-two short-chain carboxylic acids, alcohols, and esters on microbial growth of Escherichia coli MG1655 under anaerobic conditions. We analyzed toxic efects of these metabo- lites on E. coli health, quantifed by growth rate and cell mass, as a function of metabolite types, concentrations, and physiochemical properties including carbon number, chemical functional group, chain branching feature, energy density, total surface area, and hydrophobicity. Strain characterization revealed that these metabolites exert distinct toxic efects on E. coli health. We found that higher concentrations and/or carbon numbers of metabolites cause more severe growth inhibition. For the same carbon numbers and metabolite concentrations, we discovered that branched chain metabolites are less toxic than the linear chain ones. Remarkably, shorter alkyl esters (e.g., ethyl butyrate) appear less toxic than longer alkyl esters (e.g., butyl acetate). Regardless of metabolites, hydrophobicity of a metabolite, gov- erned by its physiochemical properties, strongly correlates with the metabolite’s toxic efect on E. coli health. -

BLUE BOOK 1 Methyl Acetate CIR EXPERT PANEL MEETING
BLUE BOOK 1 Methyl Acetate CIR EXPERT PANEL MEETING AUGUST 30-31, 2010 Memorandum To: CIR Expert Panel Members and Liaisons From: Bart Heldreth Ph.D., Chemist Date: July 30, 2010 Subject: Draft Final Report of Methyl Acetate, Simple Alkyl Acetate Esters, Acetic Acid and its Salts as used in Cosmetics . This review includes Methyl Acetate and the following acetate esters, relevant metabolites and acetate salts: Propyl Acetate, Isopropyl Acetate, t-Butyl Acetate, Isobutyl Acetate, Butoxyethyl Acetate, Nonyl Acetate, Myristyl Acetate, Cetyl Acetate, Stearyl Acetate, Isostearyl Acetate, Acetic Acid, Sodium Acetate, Potassium Acetate, Magnesium Acetate, Calcium Acetate, Zinc Acetate, Propyl Alcohol, and Isopropyl Alcohol. At the June 2010 meeting, the Panel reviewed information submitted in response to an insufficient data announcement for HRIPT data for Cetyl Acetate at the highest concentration of use (lipstick). On reviewing the data in the report, evaluating the newly available unpublished studies and assessing the newly added ingredients, the Panel determined that the data are now sufficient, and issued a Tentative Report, with a safe as used conclusion. Included in this report are Research Institute for Fragrance Materials (RIFM) sponsored toxicity studies on Methyl Acetate and Propyl Acetate, which were provided in “wave 2” at the June Panel Meeting but are now incorporated in full. The Tentative Report was issued for a 60 day comment period (60 days as of the August panel meeting start date). The Panel should now review the Draft Final Report, confirm the conclusion of safe, and issue a Final Report. All of the materials are in the Panel book as well as in the URL for this meeting's web page http://www.cir- safety.org/aug10.shtml. -

(2-Methoxymethylethoxy)-, Acetate (CASRN 88917-22-0) (DPMA) Final Designation
Supporting Information for Low-Priority Substance Propanol, 1(or 2)-(2-Methoxymethylethoxy)-, Acetate (CASRN 88917-22-0) (DPMA) Final Designation February 20, 2020 Office of Pollution Prevention and Toxics U.S. Environmental Protection Agency 1200 Pennsylvania Avenue Washington, DC 20460 Contents 1. Introduction ................................................................................................................................................................ 1 2. Background on Dipropylene Glycol Methyl Ether Acetate ..................................................................................... 3 3. Physical-Chemical Properties ................................................................................................................................... 4 3.1 References ...................................................................................................................................................... 6 4. Relevant Assessment History ................................................................................................................................... 7 5. Conditions of Use ....................................................................................................................................................... 8 6. Hazard Characterization .......................................................................................................................................... 12 6.1 Human Health Hazard .................................................................................................................................. -

A Study of the Reversing of Relative Volatilities by Extractive Distillation
A study of the reversing of relative volatilities by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Chemical Engineering Montana State University © Copyright by An-I Yeh (1986) Abstract: The separation of the close-boiling mixture, m-xylene-o-xylene; three binary azeotropes: ethanol-water, methyl acetate-methanol, acetone-methanol, and four ternary azeotropes: n-propyl acetate-n-propanol-water, isopropyl acetate-isopropanol-water, n—butyl acetate-n- butanol-water, isobutyl acetate-isobutanol-water has been enhanced by extractive distillation. The azeotropes have been negated and the relative volatilities of key components have been reversed by the agents used. The plot of polar interaction versus hydrogen bonding, called polarity diagram, was used to compare the affinity of agents for key components. Thus the key component which will be the overhead product can be predicted. The three solubility parameters were used to describe the intermolecular forces occurring between agents and key components in extractive distillation. The MOSCED model was used to calculate the activity coefficients of the key components using the properties of the pure compounds. The calculated values fitted the experimental data well. The advantage of this model was to calculate the. relative volatilities of key components in the presence of the agent using the properties of pure compounds instead of using the properties of mixtures. Temperature inversion, where the overhead temperature was higher than the stillpot temperature, was observed for the acetone-methanol system when ketones were used as the agents. The data showed that the temperature inversion could be caused by the dissolving of the vapor of key components in the liquid agents. -

VAPOR-LIQUID EQUILIBRIUM DATA COLLECTION Chemistry Data Series
J. Gmehling U. Onken VAPOR-LIQUID EQUILIBRIUM DATA COLLECTION Esters Supplement 2 Chemistry Data Series Vol. I, Part 5b Published by DECHEMA Gesellschaft fur Chemische Technik und Biotechnologie e. V. Executive Editor: Gerhard Kreysa Bibliographic information published by Die Deutsche Bibliothek Die Deutsche Bibliothek lists this publication in the Deutsche Nationalbibliographie; de tailed bibliographic data is available on the Internet at http://dnb.ddb.de ISBN: 3-89746-041-6 © DECH EMA Gesellschaft fOr Chemische Te chnik und Biotechnologie e. V. Postfach 15 01 04, D-60061 Frankfurt am Main, Germany, 2002 Dieses Werk ist urheberrechtlich geschutzt. Alle Rechte, auch die der Obersetzung, des Nachdrucks und der Vervielfi:Htigung des Buches oder Teilen daraus sind vorbehalten. Kein Teil des Werkes darf ohne schriftliche Genehmigung der DECHEMA in irgendeiner Form (Fotokopie, Mikrofilm oder einem anderen Verfahren), auch nicht fOr Zwecke der Un terrichtsgestaltung, reproduziert oder unter Verwendung elektronischer Systeme verarbei tet, vervielfaltigt oder verbreitet werden. Die Herausgeber Obernehmen fOr die Richtigkeit und Vollstandigkeit der publizierten Daten keinerlei Gewahrleistung. This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, including those of translation, reprinting, reproduction by photo copying machine or similar means. No partof this work may be reproduced, processed or distributed in any form, not even for teaching purposes - by photocopying, microfilm or other processes, or implemented in electronic information storage and retrieval systems - without the written permission of the publishers. The publishers accept no liability for the accuracy and completeness of the published data. This volume of the Chemistry Data Series was printed using acid-free paper. -

Experiment 5 Synthesis of Esters Using Acetic Anhydride1
EXPERIMENT 5 SYNTHESIS OF ESTERS USING ACETIC ANHYDRIDE1 Materials Needed • 2.0 mL of an alcohol to be chosen from the following: 1-propanol (n-propyl alcohol), 3-methyl-1 -butanol (isoamyl alcohol, isopentyl alcohol), 1-octanol (n-octyl alcohol), phenylmethanol (benzyl alcohol) • 2-3 mL acetic anhydride • 3 drops concentrated sulfuric acid • saturated aqueous sodium chloride (sat NaCl(aq)) • saturated aqueous sodium bicarbonate (NaHCO3(aq)) • anhydrous calcium chloride pellets (CaCl2(s)) • 1 very large test tube, several small test tubes, 1 screw-cap vial, Pasteur Pipettes Additional Reading Assignment Denniston, Chapter 15.2-15.3 (especially p 446) INTRODUCTION A carboxylic acid and an alcohol react in the presence of an acid catalyst to form an ester and water as shown in equation 1. This reaction, termed Fischer esterification in honor of its discoverer, can be used to prepare a variety of esters. O H+(cat) O R C OH HOR' R C OR' H2O (1) carboxylic acid alcohol ester water side product There are problems with this reaction however. The esterification reaction is reversible with an equilibrium constant that favors the products only slightly. Excess of either the carboxylic acid or the alcohol must be used to drive the equilibrium to the right (Le Chatelier's Principle) in order to get a decent yield of the ester product. The reaction is also rather slow (even at elevated temperatures with the acid catalyst added), too slow to allow us to carry it out in a two-hour laboratory period. Esters of acetic acid (i.e., alkyl acetates) can be prepared in a more efficient manner by using acetic anhydride (rather than acetic acid) as the non-alcohol reactant (eq 2). -

Solvent Screening Using Unifac Method to Investigate the Deodorization of Soybean Oil Using a Liquid Solvent
SOLVENT SCREENING USING UNIFAC METHOD TO INVESTIGATE THE DEODORIZATION OF SOYBEAN OIL USING A LIQUID SOLVENT P. O. B. HOMRICH, R. CERIANI Universidade Estadual de Campinas, Faculdade de Engenharia Química, Departamento de Desenvolvimento de Processos e Produtos E-mail para contato: [email protected] ABSTRACT – Removal of secondary oxidation products from edible oils, currently achieved by steam stripping in the deodorization process, may be conducted under mild conditions with a liquid solvent. Solvent screening can be used to determine more suitable solvents for removing undesirable compounds. In order to investigate the removal of n- hexanal, 2,4-decadienal and hexanoic acid, odor compounds of soybean oil, 16 solvents with low toxicity were preselected by using the qualitative method of Cusack et al. (1991). Solubility data of pseudoternary (diluent + solute + solvent) and binary (solvent + solvent) systems were predicted using UNIFAC at 298.15 K and under 1 atm in Aspen Plus. Two versions of UNIFAC were considered, namely, Magnussen et al. (1981) and Hirata et al. (2013). This last version is specifically adjusted for lipid technology systems. In general, the results indicated that 7 solvents presented a separation region (type I or II), with different mutual solubility for the solvent/diluent pair. In addition, a partial miscibility were verified for three binary systems of solvents and water. This simulated screening was a guidance for sets of experiments involving liquid-liquid equilibria under investigation currently in our lab. 1. INTRODUCTION The deodorization of vegetable oils causes various deleterious consequences, such as the removal of nutraceutical compounds, the degradation of triacylglycerol, the undesirable reactions of oxidation and cis-trans isomerization, and the 3-MCPD (3-monochloropropane-1,2-diol) contaminant formation (Suliman and Jiang, 2013; Szydłowska-Czerniak et al., 2011; Ermacora and Hrncirik, 2014). -
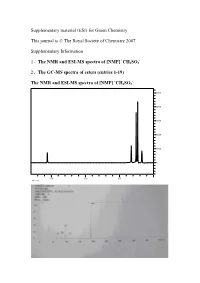
And the GC-MS
Supplementary material (ESI) for Green Chemistry This journal is © The Royal Society of Chemistry 2007 Supplementary Information + - 1、The NMR and ESI-MS spectra of [NMP] CH3SO3 2、The GC-MS spectra of esters (entries 1-19) + - The NMR and ESI-MS spectra of [NMP] CH3SO3 5000 4000 3000 2000 1000 0 15.0 10.0 5.0 ppm (t1) The GC-MS spectra of esters (entries 1-19) (1) The GC-MS spectrum of propyl acetate 0.69min, solvent of chloroform; 0.97min, propyl acetate The MS spectra of propyl acetate (0.97min) (2) The GC-MS spectrum of isopropyl acetate 1.98min, isopropyl acetate The MS spectrum of isopropyl acetate (1.98min) (3) The GC-MS spectrum of butyl acetate 2.90min, butyl acetate 4(ZHANG) #665 RT: 3.61 AV: 1 NL: 2.56E7 T: + c Full ms [ 14.00-400.00] 72.85 100 90 80 70 85.85 41.88 60 50 40 Relative Abundance 57.88 30 20 100.93 10 135.97154.12 207.58 253.45 280.90 355.76 0 50 100 150 200 250 300 350 400 m/z The MS spectrum of butyl acetate (2.90min) (4) The GC-MS spectrum of isopentyl acetate 2.20min, isopentyl acetate 3(ZHANG) #405 RT: 2.98 AV: 1 NL: 2.71E7 T: + c Full ms [ 14.00-400.00] 86.89 100 90 80 70 60 41.86 50 40 85.90 Relative Abundance Relative 57.85 30 20 69.89 10 87.91 88.94 121.16 158.00 207.11 226.90 280.98 314.33 356.16 0 50 100 150 200 250 300 350 400 m/z The MS spectrum of isopentyl acetate (2.2min) (5) The GC-MS spectrum of pentyl acetate 3.54min, pentyl acetate The MS spectrum of pentyl acetate (3.54min) (6) The GC-MS spectrum of hexyl acetate 4.15min, hexyl acetate 1 #920 RT: 3.08 AV: 1 NL: 5.24E7 T: + c Full ms [ 45.00-400.00] -

Prediction of Vapor-Liquid Equilibria with Chemical Reaction by Analytical Solutions of Groups
PREDICTION OF VAPOR-LIQUID EQUILIBRIA WITH CHEMICAL REACTION BY ANALYTICAL SOLUTIONS OF GROUPS Katsumi TOCHIGI, Shigeki MINAMIand Kazuo KOJIMA Department of Industrial Chemistry, Nihon University, Tokyo 101 To discuss prediction of vapor-liquid equilibria with esterification on the basis of the ASOG model, infinite-dilution binary activity coefficients were measured for five systems in the 40°- 100°Crange by an ebulliometric method. The systems measured are methyl acetate-»-heptane, ethyl acetate-ethanol, ethyl acetate-water, acetic acid-w-heptane, and acetic acid-ethyl acetate. The group Wilson parameters for any system made up of CH2, OH, COO, COOHgroups were determined in the 40°-100°C range by infinite-dilution activity coefficients. Vapor-liquid equilib- ria predicted for three quaternary esterification systems and for 26 binary and ternary systems involving alcohols, water, paraffins, esters, and carboxylic acids agreed fairly well with observed values. Introduction liquid equilibria with esterification by ASOGmodel. Extensive studies of experimental and theoretical madeHere, upthe ofgroupCH2,WilsonOH, COO,parametersCOOHfor groupsany systemwere aspects of vapor-liquid equilibria have been under- determined first in the 40°-100°C range by the ebullio- taken in the past, though mostly on systems in which metric method. Next, by applying the group Wilson no chemical reaction occurs. Apparently, few studies parameters, prediction of vapor-liquid and vapor- of vapor-liquid equilibria of systems in which chemical liquid-liquid equilibria was made for 22 binary reaction occurs in liquid phase, such as esterification, systems constituted by CH2-COO, CH2-OH-COO, have been made. Komatsu, Hirata et al.11~U) ob- CH2-OH-COOH, CH2-COO-COOHgroups and four served vapor-liquid equilibria of quaternary systems, ternary systems constituted by CH2-OH-COOgroups. -

Public Comment No. 1-NFPA 497-2019 [ Section No
National Fire Protection Association Report https://submittals.nfpa.org/TerraViewWeb/ContentFetcher?commentPar... Public Comment No. 1-NFPA 497-2019 [ Section No. 4.4.2 [Excluding any Sub-Sections] ] 1 of 15 12/10/2019, 1:59 PM National Fire Protection Association Report https://submittals.nfpa.org/TerraViewWeb/ContentFetcher?commentPar... An alphabetical listing of selected combustible materials, with their group classification and relevant physical properties, is provided in Table 4.4.2. Table 4.4.2 Selected Chemicals Vapor Class I Flash Vapor Clas AIT Density Chemical CAS No. Division a Point %LFL %UFL b Zon Type (°C) (Air = Pressure Group (°C) 1) (mm Hg) Grou Acetaldehyde 75-07-0 Cd I -38 175 4.0 60.0 1.5 874.9 IIA Acetic Acid 64-19-7 Dd II 39 426 19.9 2.1 15.6 IIA Acetic Acid- tert-Butyl 540-88-5 D II 1.7 9.8 4.0 40.6 Ester Acetic Anhydride 108-24-7 D II 49 316 2.7 10.3 3.5 4.9 IIA Acetone 67-64-1 Dd I –20 465 2.5 12.8 2.0 230.7 IIA Acetone Cyanohydrin 75-86-5 D IIIA 74 688 2.2 12.0 2.9 0.3 Acetonitrile 75-05-8 D I 6 524 3.0 16.0 1.4 91.1 IIA Acetylene 74-86-2 Ad GAS 305 2.5 100 0.9 36600 IIC Acrolein (Inhibited) 107-02-8 B(C)d I 235 2.8 31.0 1.9 274.1 IIB Acrylic Acid 79-10-7 D II 54 438 2.4 8.0 2.5 4.3 IIB Acrylonitrile 107-13-1 Dd I 0 481 3 17 1.8 108.5 IIB Adiponitrile 111-69-3 D IIIA 93 550 1.0 0.002 Allyl Alcohol 107-18-6 Cd I 22 378 2.5 18.0 2.0 25.4 IIB Allyl Chloride 107-05-1 D I -32 485 2.9 11.1 2.6 366 IIA Allyl Glycidyl Ether 106-92-3 B(C)e II 57 3.9 Alpha-Methyl Styrene 98-83-9 D II 574 0.8 11.0 4.1 2.7 n-Amyl Acetate -

40 CFR Ch. I (7–1–14 Edition) Pt. 59, Subpt. E, Table 2A
Pt. 59, Subpt. E, Table 2A 40 CFR Ch. I (7–1–14 Edition) Reactivity limit Coating category Category code a (g O3/g product) Corrosion Resistant Brass, Bronze, or Copper Coatings ................................................ CRB 1.80 Exact Match Finish—Engine Enamel ............................................................................... EEE 1.70 Exact Match Finish—Automotive ..................................................................................... EFA 1.50 Exact Match Finish—Industrial ......................................................................................... EFI 2.05 Floral Sprays .................................................................................................................... FSP 1.70 Glass Coatings ................................................................................................................. GCP 1.40 High Temperature Coatings ............................................................................................. HTC 1.85 Hobby/Model/Craft Coatings, Enamel .............................................................................. HME 1.45 Hobby/Model/Craft Coatings, Lacquer ............................................................................. HML 2.70 Hobby/Model/Craft Coatings, Clear or Metallic ................................................................ HMC 1.60 Marine Spar Varnishes ..................................................................................................... MSV 0.90 Photograph Coatings .......................................................................................................