Stories from Physics 5
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biochemical Thermodynamics
Biochemical Thermodynamics Biochemical Thermodynamics By Juan S. Jiménez Biochemical Thermodynamics By Juan S. Jiménez This book first published 2020 Cambridge Scholars Publishing Lady Stephenson Library, Newcastle upon Tyne, NE6 2PA, UK British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Copyright © 2020 by Juan S. Jiménez All rights for this book reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner. ISBN (10): 1-5275-5359-0 ISBN (13): 978-1-5275-5359-0 To the memory of Brígida and Francisco Jiménez CONTENTS PREFACE ..................................................................................................... x CHAPTER 1 .................................................................................................. 1 INTRODUCTION 1.1 The Atomic Theory of John Dalton and the Hypothesis of Amedeo Avogadro 1.2 The Mole and Avogadro’s Number 1.3 The ideal gas model 1.4 The Periodic Table and Initial Atomic Theories 1.5 The Hydrogen Atom and the Schrödinger Equation 1.6 Atomic Structure 1.7 Molecules CHAPTER 2 ................................................................................................ 35 ENTROPY 2.1 Systems, Properties and States 2.2 The First Law of Thermodynamics 2.3 Enthalpy 2.4 Reversible changes 2.5 The Second Law of Thermodynamics 2.6 A Particle in a One-dimensional Box 2.7 Quantum States 2.8 The Boltzmann Equation CHAPTER 3 ................................................................................................ 63 THE CHEMICAL EQUILIBRIUM 3.1 The Gibbs Function 3.2 The Chemical Potential 3.3 Chemical Equilibrium 3.4 Model Systems 3.5 The Equilibrium Constant for Chemical Reactions between Gases. -

Amedeo Avogadro
Amedeo Avogadro ALSO LISTED IN Physicists FAMOUS AS Chemist and Physicist NATIONALITY Italian Famous Italian Men RELIGION Roman Catholic BORN ON 09 August 1776 AD Famous 9th August Birthdays ZODIAC SIGN Leo Leo Men BORN IN Turin, Italy DIED ON 09 July 1856 AD PLACE OF DEATH Turin, Italy FATHER Filippo Avogadro MOTHER Anna Maria Vercellone SPOUSE: Felicita Mazzé Lorenzo Romano Amedeo Carlo Avogadro de Quaregna e di Cerreto, more popularly known as Amedeo Avogadro was born on August 9, 1776, in Turin, Italy. He was a gifted physicist and chemist who proposed the molecular theory, which is more popularly known as ‘Avogadro’s Law’. Although he earned a doctorate in ecclesiastical law, he developed a passion for studying mathematics and physics. He then gave up his career in law and pursued a career teaching natural physics at the Royal College of Vercelli. Years later, he was offered the chair of mathematical physics at the University of Turin. Avogadro conducted experiments in both physics and chemistry using mathematics as a basis for his findings. His hypothesis, known as the ‘Avogadro’s Law’ is recognized all over the world. He also published many works during his lifetime. The number 6.02214199 x 10^23 is named as Avogadro’s number to honor him for his contribution in molecular theory. Read on to know more about this great physicist and chemist. Read more at http://www.thefamouspeople.com/profiles/amadeo-avogadro- 532.php#09s5lE6QjEOdfSro.99 Career After studying philosophy in 1789, Amedeo Avogadro graduated in jurisprudence in 1792 and earned his doctorate in ecclesiastical law in 1796. -

Chemistry in Italy During Late 18Th and 19Th Centuries
CHEMISTRY IN ITALY DURING LATE 18TH AND 19TH CENTURIES Ignazio Renato Bellobono, CSci, CChem, FRSC LASA, Department of Physics, University of Milan. e-mail add ress : i.bell obon o@ti scali.it LASA, Dept.Dept. ofPhysics, Physics, University of Milan The birth of Electrochemistry Luigi Galvani, Alessandro Volta, and Luigi Valentino Brugnatelli From Chemistry to Radiochemistry The birth of Chemistry and Periodic Table Amedeo Avogadro and Stanislao Cannizzaro Contributions to Organic Chemistry LASA, Dept.Dept. ofPhysics, Physics, University of Milan 1737 At the Faculty of Medicine of the Bologna University, the first chair of Chemistry is establishedestablished,,andandassigned to Jacopo Bartolomeo BECCARI (1692-1766). He studied phosphorescence and the action of light on silver halides 1776 In some marshes of the Lago Maggiore, near AngeraAngera,, Alessandro VOLTA ((17451745--18271827),),hi gh school teacher of physics in Como, individuates a flammable gas, which he calls aria infiammabile. Methane is thus discovereddiscovered.. Two years laterlater,,heheis assignedassigned,,asas professor of experimental phihysicscs,,toto the UiUniversi ty of PiPavia LASA, DtDept. of PhPhys icscs,, University of Milan 1778 In aletter a letter to Horace Bénédict de Saussure, aaSwissSwiss naturalist, VOLTA introduces, beneath that of electrical capacitycapacity,, the fundamental concept of tensione elettrica (electrical tension), exactly the name that CITCE recommended for the difference of potential in an electrochemical cell. 17901790--17911791 VOLTA anticipatesanticipates,,bybyabout 10 yearsyears,,thethe GAYGAY--LUSSACLUSSAC linear de ppyendency of gas volume on tem pp,erature, at constant pressurepressure,,andandafew a fewyears later ((17951795)) anticipatesanticipates,,byby about 6years 6 years,,thethe soso--calledcalled John Dalton’s rules ((18011801))ononvapour pressure LASA, Dept.Dept. -

What Would Happen to Our Globes on the Globe of Mars?
FEATURE Globes in space: What would happen to our globes on the globe of Mars? BY KATHERINE MCVEIGH AND TOMAS BURKE Many films have been made regarding life on alternative planets. With the Mars One mission approaching in 2023, there are high expectations regarding future interplanetary travel. The authors provide an ophthalmology perspective on what could happen to our eyes if exposed to the atmosphere of Mars. n 1990, Arnold Schwarzenegger starred in Total Recall [1], a film where he finds himself on Mars. Damage to his Ispacesuit and subsequent exposure to the environment on Mars results in excessive bodily swelling, along with extensive proptosis whereby the globes extend anteriorly beyond the eyelids as his optic nerves are undoubtedly stretched to their maximal capacity. From an ophthalmological perspective, the interpretation of the impact of atmospheric exposure is intriguing. With the anticipated Mars One mission launch in 2023 aiming to establish a habitable settlement in a hostile environment, we ask if what happened is an accurate depiction of what could be expected to happen to us and our ophthalmic globes on that other globe? The atmosphere on Mars has an O2 level of 0.13% and CO2 level of 95%, and is very Armstrong limit, meaning that exposure to thin, approximately 1% of that on Earth at Tense oedematous soft tissue swelling of sea level [2]. This is believed to be a result the environment would result in a prompt the lids will then make it challenging to of the loss of the magnetosphere four and severe dehydration from the mucosal open the lids. -

NEAR of the 21 Lunar Landings, 19—All of the U.S
Copyrights Prof Marko Popovic 2021 NEAR Of the 21 lunar landings, 19—all of the U.S. and Russian landings—occurred between 1966 and 1976. Then humanity took a 37-year break from landing on the moon before China achieved its first lunar touchdown in 2013. Take a look at the first 21 successful lunar landings on this interactive map https://www.smithsonianmag.com/science-nature/interactive-map-shows-all-21-successful-moon-landings-180972687/ Moon 1 The near side of the Moon with the major maria (singular mare, vocalized mar-ray) and lunar craters identified. Maria means "seas" in Latin. The maria are basaltic lava plains: i.e., the frozen seas of lava from lava flows. The maria cover ∼ 16% (30%) of the lunar surface (near side). Light areas are Lunar Highlands exhibiting more impact craters than Maria. The far side is pocked by ancient craters, mountains and rugged terrain, largely devoid of the smooth maria we see on the near side. The Lunar Reconnaissance Orbiter Moon 2 is a NASA robotic spacecraft currently orbiting the Moon in an eccentric polar mapping orbit. LRO data is essential for planning NASA's future human and robotic missions to the Moon. Launch date: June 18, 2009 Orbital period: 2 hours Orbit height: 31 mi Speed on orbit: 0.9942 miles/s Cost: 504 million USD (2009) The Moon is covered with a gently rolling layer of powdery soil with scattered rocks called the regolith; it is made from debris blasted out of the Lunar craters by the meteor impacts that created them. -

Professor Name Class Student ID Number Name Problem Points
2019 Fall Semester Midterm Examination For General Chemistry I Date: Oct 23 (Wed), Time Limit: 19:00 ~ 21:00 Write neatly in the spaces provided below each question; print your Student ID in the upper right corner of every page. Professor Class Student I.D. Number Name Name Problem points Problem points TOTAL pts 1 /8 6 /10 2 /10 7 /10 3 /10 8 /8 /100 4 /10 9 /10 5 /10 10 /14 ** This paper consists of 10 pages with 10 problems (pages 12 - 14: Equations, constants & periodic table, page 15: form for claiming credit). Please check all page numbers before taking the exam. Write down your work and answers in the Answer sheet. Please the numerical value of your answer with the appropriate unit when applicable. You will get 30% deduction for a missing unit. NOTICE: SCHEDULES on RETURN and CLAIM of the MARKED EXAM PAPER. (채점 답안지 분배 및 이의신청 일정) 1. Period, Location, and Procedure 1) Return and Claim Period: Oct 28 (Mon, 7:00 ~ 8:00 p.m.) 2) Location: Room for quiz sessions 3) Procedure: Rule 1: Students cannot bring their own writing tools into the room. (Use a pen only provided by TA) Rule 2: Whether you have made a claim or not, you must submit the paper back to TA. (Do not go out of the room with it) If you have any claims on it, you can submit the claim paper with your opinion. After writing your opinions on the claim form, please staple it to your mid-term paper. -

Where in the Air Classroom Activity Educator Guide 2 | WHERE in the AIR CLASSROOM ACTIVITY EDUCATOR GUIDE
National Aeronautics and Space Administration STEM ACTIVITY: Where in the Air Classroom Activity Educator Guide www.nasa.gov 2 | WHERE IN THE AIR CLASSROOM ACTIVITY EDUCATOR GUIDE OVERVIEW In this lesson, students learn about the layers of the atmosphere as well as what things can be found in each layer. Objectives Students will be able to: • Identify the layers of the atmosphere, including their location relative to the other layers • Determine in which layer of the atmosphere different objects can be found Standards Materials Worksheet – one per student Next Generation Science Standards • Disciplinary Core Ideas Informational sheets – one per group or student • MS-ESS2 Earth’s systems • Crosscutting Concepts • Systems and system models Science and Engineering Practices • Developing and using models Preparation Other Resources • Print out a worksheet for each student and “Earth’s Atmospheric Layers” diagram, available at: enough informational sheets so each student https://www.nasa.gov/mission_pages/sunearth/science/ will get a copy of the sheet describing their atmosphere-layers2.html group’s object. • The object descriptions in the informational sheets vary in complexity, allowing reading variation for students. WHERE IN THE AIR CLASSROOM ACTIVITY EDUCATOR GUIDE | 3 Steps 1. Use a warm-up or other method to teach (or review) the layers of the atmosphere. A search of the internet will provide you with many possible activities or videos that can be used to engage students. This information can be presented in conjunction with Earth’s other spheres (cryosphere, hydrosphere, biosphere, etc.). 2. Divide the students into groups of two or three. Or, depending on class size and composition, students can work as individuals instead of in small groups. -

Avogadro's Constant
Avogadro's Constant Nancy Eisenmenger Question Artem, 8th grade How \Avogadro constant" was invented and how scientists calculated it for the first time? Answer What is Avogadro's Constant? • Avogadros constant, NA: the number of particles in a mole • mole: The number of Carbon-12 atoms in 0.012 kg of Carbon-12 23 −1 • NA = 6:02214129(27) × 10 mol How was Avogadro's Constant Invented? Avogadro's constant was invented because scientists were learning about measuring matter and wanted a way to relate the microscopic to the macroscopic (e.g. how many particles (atoms, molecules, etc.) were in a sample of matter). The first scientists to see a need for Avogadro's constant were studying gases and how they behave under different temperatures and pressures. Amedeo Avogadro did not invent the constant, but he did state that all gases with the same volume, pressure, and temperature contained the same number of gas particles, which turned out to be very important to the development of our understanding of the principles of chemistry and physics. The constant was first calculated by Johann Josef Loschmidt, a German scientist, in 1865. He actually calculated the Loschmidt number, a constant that measures the same thing as Avogadro's number, but in different units (ideal gas particles per cubic meter at 0◦C and 1 atm). When converted to the same units, his number was off by about a factor of 10 from Avogadro's number. That may sound like a lot, but given the tools he had to work with for both theory and experiment and the magnitude of the number (∼ 1023), that is an impressively close estimate. -

Contacts: Isabel Morales, Museum of Science and Industry, (773) 947-6003 Renee Mailhiot, Museum of Science and Industry, (773) 947-3133
Contacts: Isabel Morales, Museum of Science and Industry, (773) 947-6003 Renee Mailhiot, Museum of Science and Industry, (773) 947-3133 A GLOSSARY OF TERMS Aerodynamics: The study of the properties of moving air, particularly of the interaction between the air and solid bodies moving through it. Afterburner: An auxiliary burner fitted to the exhaust system of a turbojet engine to increase thrust. Airfoil: A structure with curved surfaces designed to give the most favorable ratio of lift to drag in flight, used as the basic form of the wings, fins and horizontal stabilizer of most aircraft. Armstrong limit: The altitude that produces an atmospheric pressure so low (0.0618 atmosphere or 6.3 kPa [1.9 in Hg]) that water boils at the normal temperature of the human body: 37°C (98.6°F). The saliva in your mouth would boil if you were not wearing a pressure suit at this altitude. Death would occur within minutes from exposure to the vacuum. Autothrottle: The autopilot function that increases or decreases engine power,typically on larger aircraft. Avatar: An icon or figure representing a particular person in computer games, Internet forums, etc. Aerospace: The branch of technology and industry concerned with both aviation and space flight. Carbon fiber: Thin, strong, crystalline filaments of carbon, used as a strengthening material, especially in resins and ceramics. Ceres: A dwarf planet that orbits within the asteroid belt and the largest asteroid in the solar system. Chinook: The Boeing CH-47 Chinook is an American twin-engine, tandem-rotor heavy-lift helicopter. CST-100: The crew capsule spacecraft designed by Boeing in collaboration with Bigelow Aerospace for NASA's Commercial Crew Development program. -

The Mole and Avogadro's Number
The Mole and Avogadro's Number The name mole (German Mol) is attributed to Wilhelm Ostwald who introduced the concept in the year 1902. It is an abbreviation for molecule (German Molekül), which is in turn derived from Latin moles "mass, massive structure". (From the Wikipedia article on the mole unit.) A good site that introduces the mole concept and includes sample calculations and practice problems can be found here, from John Park's excellent ChemTeam site. For some interesting background on Avogadro's number, see here. By T.A. Furtsch, Tennessee Technological University, Cookeville, TN. Don't miss the interview with Count Amedeo Avogadro and his wife the Countess Felicita, located here (thanks to Kory Tonouchi, Roosevelt High, Honolulu, HI). Avogadro's 1811 publication, "Essay on a Manner of Determining the Relative Masses of the Elementary Molecules of Bodies, and the Proportions in Which They Enter into These Compounds", may be found here (thanks to Carmen Giunta, Le Moyne College, Syracuse, NY). A fun "mole" page to visit is here. It is a collection of student projects from Carondelet High School. Back in '98 they had a mole mystery described here. Did you know we have a mole day? Find out about it here. And for some corny mole jokes, don't miss the Dictionary of Mole Day Terms & Jokes here! Introduction A mole of objects contains Avogadro's number, 6.022 X 1023, objects. Just as a dozen apples is 12 apples, a mole of apples is 6.022 X 1023 apples. A mole of iron atoms is 6.022 X 1023 iron atoms. -
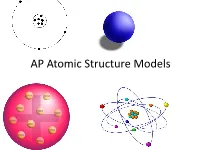
AP Atomic Structure Models What Is a Model?
AP Atomic Structure Models What is a Model? On a scrap piece of paper, write down your definition of a model with at least two examples. A model is a representation of an object, idea, action, or concept. Models A model in science is usually an approximation of the “real thing.” This means that all models have simplifications that may lead to omitted information. Models When a model is selected for a specific purpose, it should be able to help predict a “behavior.” The Four Historical Models of the Atom 1. Particle model 2. Plum Pudding model 3. Rutherford model 4. Bohr model Modern Model Quantum Mechanical Model Why do we have so many historical models? Are some of them still useful? When we initially began the process of determining the structure of the atom, we first had to overcome the prevailing scientific concept that matter was continuous. This idea of continuous matter was from Aristotle. (a Greek philosopher 384-322 B.C.E.) Particle Model John Dalton’s four ideas about the particle nature of matter helped push forward the acceptance of the first model. 1. All elements are composed of tiny indivisible particles called atoms. 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. John Dalton 3. Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction. -

Where in the Air Classroom Activity Student Guide.Pdf
National Aeronautics and Space Administration STEM ACTIVITY: Where in the Air Classroom Activity Student Guide www.nasa.gov 2 | WHERE IN THE AIR CLASSROOM ACTIVITY STUDENT GUIDE OVERVIEW In this lesson, you will learn about the layers of the atmosphere as well as some of the things that can be found in each layer. Objectives Students will be able to: • Identify the layers of the atmosphere, including their location relative to the other layers • Determine in which layer of the atmosphere different objects can be found Directions Complete the following worksheet. 1. Part 1: You will be given an informational sheet which describes the object that you are to learn about. Read through this sheet to become the class expert. As you read, fill in the information on part 1 of the worksheet. When you have finished, use this information to complete the portion of the table in part 2 about your object. Prepare to share this information with the class. 2. Part 2: Each group will take turns sharing the information they gathered about their object. As they teach you about their object, use the information they provide to complete the rest of the table in part 2 of the worksheet. 3. Part 3: On the chart in part 3 of the worksheet, fill in the names for each layer of the atmosphere. Then, write the name of each object in its appropriate layer. If time permits, add a small drawing for each object. WHERE IN THE AIR CLASSROOM ACTIVITY STUDENT GUIDE | 3 NAME: “Where in the Air?” Student Worksheet PART 1 You are going to become an expert on one object found in Earth’s atmosphere.