Migraine As a Stroke Mimic and As a Stroke Chameleon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fibromyalgia and Chronic Fatigue
Fibromyalgia and Chronic Fatigue Paul G. Swingle, Ph.D., R. Psych. My previous radio program and my present webcast program are both entitled “It’s all in your head!” How I came up with this title was listening to the discouraged patients that I routinely treated, and still treat, who have been told, by the doctors they have consulted, that the pain, discomfort, distress, debilitating fatigue, sleep disturbance and depression that they endure is “all in your head.” What this remark is meant to imply, of course, is that there is no physical cause of the condition and that the symptoms are manifestations of psychological factors. My response to these patients is that “of course it is in your head, where else would it be?” My remark however is not disparaging but rather indicates the actual location of many of the patients symptoms. The brain controls everything so all symptoms are associated with brain activity. The remarkable effectiveness of neurotherapy for the treatment of the symptoms associated with fibromyalgia and chronic fatigue is due to the fact that the brain functioning associated with the symptoms is treated. Although this derogatory sentiment about fibromyalgia patients was widespread among health care providers in the past, it is remarkable to me that it still persists with many doctors to this day. A few days ago I was surfing the internet health news feeds and was dismayed to find an item entitled “Fibromyalgia: A mental illness?” In this item was a quote from a Canadian neurologist stating that fibromyalgia was a term waiting for a definition. -

SOS – Save Our Shoulders: a Guide for Polio Survivors
1 • Save Our Shoulders: A Guide for Polio Survivors A Guide for Polio Survivors S.O.S. Save Our Shoulders: A Guide for Polio Survivors by Jennifer Kuehl, MPT Roberta Costello, MSN, RN Janet Wechsler, PT Funding for the production of this manual was made possible by: The National Institute for Disability and Rehabilitation Research Grant #H133A000101 and The U.S. Department of the Army Grant #DAMD17-00-1-0533 Investigators: Mary Klein, PhD Mary Ann Keenan, MD Alberto Esquenazi, MD Acknowledgements We gratefully acknowledge the contributions and input provided from all of those who participated in our research. The time and effort of our participants was instrumental in the creation of this manual. Jennifer Kuehl, MPT Moss Rehabilitation Research Institute, Philadelphia Roberta Costello, MSN, RN Moss Rehabilitation Research Institute, Philadelphia Janet Wechsler, PT Moss Rehabilitation Research Institute, Philadelphia Mary Klein, PhD Moss Rehabilitation Research Institute, Philadelphia Mary Ann Keenan, MD University of Pennsylvania, Philadelphia Alberto Esquenazi, MD MossRehab Hospital, Philadelphia Cover and manual design by Ron Kalstein, MEd Albert Einstein Medical Center, Philadelphia Table of Contents 1. Introduction . .5 2. General Information About the Shoulder . .6 3. Facts About Shoulder Problems . .8 4. Treatment Options . .13 5. About Exercise . .16 6. Stretching Exercises . .19 7. Cane Stretches . .22 8. Strengthening Exercises . .25 9. Tips to Avoid Shoulder Problems . .29 10. Conclusion . .31 11. Resources . .31 The information contained within this manual is for reference only and is not a substitute for professional medical advice. Before beginning any exercise program consult your physician. Save Our Shoulders: A Guide for Polio Survivors • 4 Introduction Many polio survivors report new symptoms as they age. -

Vascular Dementia: an Overview of Current Challenges and Gaps
Stroke and Dementia: what research tells us UK Stroke Assembly 2016 JM Wardlaw University of Edinburgh NHS Lothian www.strokeassembly.org.uk #strokeassembly Definitions Stroke Dementia Sudden onset focal A syndrome where there neurological deficit which is deterioration in last more than 24 hours memory, thinking, attributable to a vascular behaviour such that the defect and has no other person is no longer able identifiable cause to perform everyday activities by themselves www.strokeassembly.org.uk #strokeassembly Stroke – the importance • Stroke, 16.9million new strokes per year, – A third are disabled and dependent – 2nd commonest cause of death – Commonest cause of dependency in adults Infarct, or ischaemic stroke Haemorrhagic stroke www.strokeassembly.org.uk #strokeassembly Cerebrovascular disease – the importance • ‘Silent’ cerebrovascular disease, even bigger issue – Up to 45% of 35.6million dementias – Cognitive impairment, mobility problems Normal White matter disease www.strokeassembly.org.uk #strokeassembly Dementia • 47.5 million people have dementia world wide • 7.7million new cases per year • Affects different people in different ways • Major cause of dependency and disability amongst older people • Alzheimer’s disease commonest cause – 60-70% of cases • Vascular dementia 2nd commonest – 25-45% of all cases www.strokeassembly.org.uk #strokeassembly Dementia: historical perspective 1800’s – Blood vessel diseases 1900’s – Alzheimers disease - dominant 1970’s – ‘Multi-infarct dementia’ – vascular 1990’s–2000’s - ‘Vascular -

Fatigue After Stroke
SPECIAL REPORT Fatigue after stroke PJ Tyrrell Fatigue is a common symptom after stroke. It is not invariably related to stroke severity & DG Smithard† and can occur in the absence of depression. It is one of the most troublesome symptoms for †Author for correspondence many patients and yet nothing is known of its causation. There are no specific treatments. William Harvey Hospital, Richard Steven’s Ward, This article assesses the available literature in the context of what is known about fatigue Ashford, Kent, in other disorders. Post-stroke fatigue may be a manifestation of sickness behavior, TN24 0LZ, UK mediated through the central effects of the cytokine interleukin-1, perhaps via effects on Tel.: +44 123 361 6214 Fax: +44 123 361 6662 glutamate neurotransmission. Possible therapeutic strategies are discussed which might be [email protected] a logical basis from which to plan randomized control trials. Following stroke, approximately a third of common and disabling symptom of Parkinson’s patients die, a third recover and a third remain disease [7,8] and of systemic lupus [9]. More than significantly disabled. Even those who recover 90% of patients with poliomyelitis develop a physically may be left with significant emotional delayed syndrome of post-myelitis fatigue [10]. and psychologic dysfunction – including anxi- Fatigue is the most prevalent symptom of ety, readjustment reactions and depression. One patients with cancer who receive radiation, cyto- common but often overlooked symptom is toxic or other therapies [11], and it may persist fatigue. This may occur soon or late after stroke, for years after the cessation of treatment [12]. -

TIA Vs CVA (STROKE)
Phone: 973.334.3443 Email: [email protected] NJPR.com TIA vs CVA (STROKE) What is the difference between a TIA and a stroke? Difference Between TIA and Stroke • Both TIA and stroke are due to poor blood supply to the brain. • Stroke is a medical emergency and it’s a life-threatening condition. • The symptoms of TIA and Stroke may be same but TIA symptoms will recover within 24 hours. TRANSIENT ISCHEMIC ATTACK ● Also known as: TIA, mini stroke 80 E. Ridgewood Avenue, 4th Floor Paramus, NJ 07652 TIA Causes ● A transient ischemic attack has the same origins as that of an ischemic stroke, the most common type of stroke. In an ischemic stroke, a clot blocks the blood supply to part of your brain. In a transient ischemic attack, unlike a stroke, the blockage is brief, and there is no permanent damage. ● The underlying cause of a TIA often is a buildup of cholesterol- containing fatty deposits called plaques (atherosclerosis) in an artery or one of its branches that supplies oxygen and nutrients to your brain. ● Plaques can decrease the blood flow through an artery or lead to the development of a clot. A blood clot moving to an artery that supplies your brain from another part of your body, most commonly from your heart, also may cause a TIA. CEREBROVASCULAR ACCIDENT/STROKE Page 2 When the brain’s blood supply is insufficient, a stroke occurs. Stroke symptoms (for example, slurring of speech or loss of function in an arm or leg) indicate a medical emergency. Without treatment, the brain cells quickly become impaired or die. -
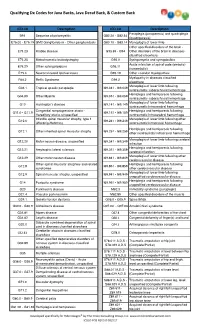
ICD-10 Backs
Qualifying Dx Codes for Java Backs, Java Decaf Back, & Custom Back ICD-10 Description ICD-10 Description Paraplegia (paraparesis) and quadriplegia B91 Sequelae of poliomyelitis G82.20 - G82.54 (quadriparesis) E75.00 - E75.19 GM2 Gangliosidosis - Other gangliosidosis G83.10 - G83.14 Monoplegia of lower limb Other specified disorders of the brain - E75.23 Krabbe disease G93.89 - G94 Other disorders of the brain in diseases classified elsewhere E75.25 Metachromatic leukodystrophy G95.0 Syringomyelia and syringobulbia Acute infarction of spinal code (embolic) E75.29 Other sphingolipidosis G95.11 (nonembolic) E75.4 Neuronal ceroid lipofuscinosis G95.19 Other vascular myelopathies Myelopathy in diseases classified F84.2 Rett's Syndrome G99.2 elsewhere Monoplegia of lower limb following G04.1 Tropical spastic paraplegia I69.041 - I69.049 nontraumatic subarachnoid hemorrhage Hemiplegia and hemiparesis following G04.89 Other Myelitis I69.051 - I69.059 nontraumatic subarachnoid hemorrhage Monoplegia of lower limb following G10 Huntington's disease I69.141 - I69.149 nontraumatic intracerebral hemorrhage Congenital nonprogressive ataxia - Hemiplegia and hemiparesis following G11.0 - G11.9 I69.151 - I69.159 Hereditary ataxia, unspecified nontraumatic intracerebral hemorrhage Infantile spinal muscular atrophy, type 1 Monoplegia of lower limb following other G12.0 I69.241 - I69.249 (Werdnig-Hoffman) nontraumatic intracranial hemorrhage Hemiplegia and hemiparesis following G12.1 Other inherited spinal muscular atrophy I69.251 - I69.259 other nontraumatic -
Cluster Headacheheadache
ClusterCluster HeadacheHeadache 1 OBJECTIVESOBJECTIVES Describe the clinical features and diagnosis of cluster headache Discuss the pathogenesis of cluster pain and autonomic features Review acute and preventive therapy Overview of new treatment horizons for refractory chronic cluster 2 IHSIHS CLASSIFICATIONCLASSIFICATION Cluster headache n Episodic type (80%) n Chronic type (20%) (Cluster period lasts for more than one year without remission or remission lasts less than 14 days) Episodic Chronic IHS Headache Classification Committee. Cephalalgia. 2004. Although the unique clinical features of cluster headache (CH) have been recognized since the 17th century, the striking periodicity was not articulated until the 1940s. The term “cluster headache” was coined in the 1950s, and since then the International Headache Society (IHS) has identified and classified two major temporal patterns of CH (1). The episodic type (ECH), by far the most common (90%), is characterized by discrete attack and remission phases. The chronic type (CCH) is defined by attacks that occur daily for more than one year without remission or with remission periods lasting less than 14 days. Cluster headache is rare (about 0.4% of the general population), and it predominates in males, although recent studies indicate that the rate in females is rising (2). Onset can occur at any age but usually begins between 30 and 50 years of age (3). In contrast to migraine headache, genetics in cluster headache is not thought to be important, although recent studies have shown a positive family history in about 7% of patients with cluster headache. When compared with prevalence of CH in the general population, first-degree relatives have about a 14-fold increased risk of developing CH. -

HEADACHES and ME/CFS INTRODUCTION Headaches Are Often Reported by People with ME/CFS
MANAGEMENT FILE the ME association by DR CHARLES SHEPHERD, our medical adviser This leaflet is based on an article which first appeared in the ME Association’s quarterly ME Essential magazine. MEA membership costs £18 a year for people living in the UK/BFPO. For contact details, see foot of this page. February 2020 HEADACHES AND ME/CFS INTRODUCTION Headaches are often reported by people with ME/CFS. They also form part of the symptom list for most diagnostic criteria for ME/CFS. But headaches can obviously have other causes – physical and psychological – as well. WHAT CAUSES HEADACHES? Doctors classify headaches as being sides of the head – as if a rubber band arteries in the head and neck. primary where there is no obvious link has been stretched around it. Common Temporal arteritis is a medical to an underlying medical problem, or causes include stress, depression, lack emergency that requires urgent treat- secondary where there is a clear link. of proper sleep, skipping meals, not ment with high-dose steroids. drinking enough fluid and alcohol. Primary headaches include: Headaches in women can be caused Secondary headaches are caused by Cluster headaches by hormonal problems – including an existing medical problem. Some of An excruciatingly painful headache taking the contraceptive pill, going these are serious, which is why you must that causes intense pain around one through the menopause, pregnancy, see your doctor if you have severe or eye. Cluster headaches are fairly rare and as part of period pain. persistent headaches. and tend to occur in clusters for a It’s also worth noting that a headache month or two, sometimes around the Common causes, which are normally is the most common symptom of same time each year. -

Chronic Fatigue Syndrome (CFS) Disease Fact Sheet Series
WISCONSIN DIVISION OF PUBLIC HEALTH Department of Health Services Chronic Fatigue Syndrome (CFS) Disease Fact Sheet Series What is chronic fatigue syndrome? Chronic fatigue syndrome (CFS) is a recently defined illness consisting of a complex of related symptoms. The most characteristic symptom is debilitating fatigue that persists for several months. What are the other symptoms of CFS? In addition to profound fatigue, some patients with CFS may complain of sore throat, slight fever, lymph node tenderness, headache, muscle and joint pain (without swelling), muscle weakness, sensitivity to light, sleep disturbances, depression, and difficulty in concentrating. Although the symptoms tend to wax and wane, the illness is generally not progressive. For most people, symptoms plateau early in the course of the illness and recur with varying degrees of severity for at least six months and sometimes for several years. What causes CFS? The cause of CFS is not yet known. Early evidence suggested that CFS might be associated with the body's response to an infection with certain viruses, however subsequent research has not shown an association between an infection with any known human pathogen and CFS. Other possible factors that have been suspected of playing a role in CFS include a dysfunction in the immune system, stress, genetic predisposition, and a patient’s psychological state. Is CFS contagious? Because the cause of CFS remains unknown, it is impossible to answer this question with certainty. However, there is no convincing evidence that the illness can be transmitted from person to person. In fact, there is no indication at this time that CFS is caused by any single recognized infectious disease agent. -
Stroke: Learn the Warning Signs and Protect Yourself Strokes Are the Leading Cause of Major Disability in the U.S
stroke: learn the warning signs and protect yourself Strokes are the leading cause of major disability in the U.S. and the second leading cause of death worldwide. With all the emphasis placed on heart disease reaches us, they’ve already been to the hospital. The and cancer as the two leading causes of death in only thing left by then is active physical therapy.” America, people often forget what ranks as third. The answer is stroke, and it can have debilitating Dr. Ray says that is why he preaches religiously effects even if you survive. to patients about lowering blood pressure and cholesterol, healthy diet and regular exercise, and On average, someone has a stroke in the U.S. every keeping any pre-existing conditions in check through 45 seconds, which equals 700,000 strokes a year. regular checkups. He goes on to say that having a Of these, about 500,000 are first-time strokes. primary care physician is vital. Strokes are so pervasive that they are the leading cause of major disability in the “All adults should speak to their doctors U.S. and the second leading cause of death about diet and controlling their blood worldwide. pressure,” Dr. Ray says. “You want to actively prevent this before it happens.” A stroke occurs when blood flow to parts of the brain is restricted or impaired, There are two basic types of stroke. The first killing off brain cells. Those cells can never is ischemic stroke caused by blood clots or regenerate, which means the damage is other particles in the blood vessels leading permanent. -

A Retrospective, Epidemiological Review of Hemiplegic Migraines in a Military Population
MILITARY MEDICINE, 00, 0/0:1, 2019 A Retrospective, Epidemiological Review of Hemiplegic Migraines in a Military Population Downloaded from https://academic.oup.com/milmed/advance-article-abstract/doi/10.1093/milmed/usz040/5382215 by AMSUS Member Access user on 22 April 2019 CPT Brian A. Moore, USAR*†; Willie J. Hale*; Paul S. Nabity†; CAPT Tyler R. Koehn, MC, USAF‡; Donald McGeary†; Lt Col Alan L. Peterson, BSC USAF (Ret.)*†§ ABSTRACT Introduction: Headaches are one of the world’s most common disabling conditions. They are also both highly prevalent and debilitating among military personnel and can have a significant impact on fitness for duty. Hemiplegic migraines are an uncommon, yet severely incapacitating, subtype of migraine with aura for which there has been a significant increase amongst US military personnel over the past decade. To date, there has not been a scientific report on hemiplegic migraine in United States military personnel. Materials and Methods: The aim of this study was to provide an overview of hemiplegic migraine, to analyze data on the incidence of hemiplegic migraine in US military service members, and to evaluate demographic factors associated with hemiplegic migraine diagnoses. First time diagnoses of hemiplegic migraine were extracted from the Defense Medical Epidemiological Database according to ICD-9 and ICD-10 codes for hemiplegic migraine. One sample Chi-Square goodness of fit tests were conducted on weighted demographic samples to determine whether significant proportional differences existed between gender, age, military grade, service component, race, and marital status. Results: From 1997 to 2007 there were no cases of hemiplegic migraine recorded in the Defense Medical Epidemiological Database. -

Central Pain: Clinical and Physiological J Neurol Neurosurg Psychiatry: First Published As 10.1136/Jnnp.61.1.62 on 1 July 1996
626Journal ofNeurology, Neurosurgery, and Psychiatry 1996;61:62-69 Central pain: clinical and physiological J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.61.1.62 on 1 July 1996. Downloaded from characteristics David Bowsher Abstract spinothalamic pathway, its relays, or projec- Objectives-To study the clinical and tions may cause central pain,' and modern pathophysiological features of central radiological techniques have tended to con- pain due to damage to the CNS. firm this.9 '" Because of this, and of recent Methods-156 patients (mostly with considerations on pathophysiology,'5 '" the ischaemic strokes, some with infarct after condition is now known as "central poststroke subarachnoid haemorrhage and other pain (CPSP)"'.'7 Stroke is not the only condi- cerebral conditions; one with bulbar and tion causing such central pains of cerebral ori- others with spinal pathology) with central gin.' lxII 2' Furthermore, identical pains occur pain have been investigated clinically and after some spinal lesions.- 22 varying numbers instrumentally with respect to quantitative somatosensory perception thresholds and autonomic Patients function. Between 1983 and 1993, 156 patients have Results-Pain onset was immediate in a been referred to me, in whom a diagnosis of minority; and from a week or two up to central pain due to a cerebral or spinal lesion six years in > 60%. For those with supra- has been made. Of these, 112 (64 (57)%'Y spinal ischaemic lesions, the median age male) had a history of a stroke episode (cere- of onset was 59; dominant and non- brovascular accident, CVA patients), includ- dominant sides were equally affected.