Latest Recommended (2018) • Fix Issues/8/Typeerror-On-Some-Of-The-Element
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
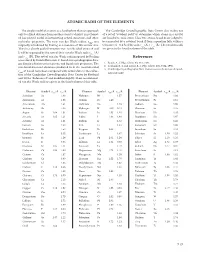
ATOMIC RADII of the ELEMENTS References
ATOMIC RADII OF THE ElEMENTS The simple model of an atom as a hard sphere that can approach The Cambridge Crystallographic Data Center also makes use only to a fixed distance from another atom to which it is not bond- of a set of “covalent radii” to determine which atoms in a crystal ed has proved useful in interpreting crystal structures and other are bonded to each other . Thus two atoms A and B are judged to molecular properties . The term van der Waals radius, rvdw, was be connected by a covalent bond if their separation falls within a originally introduced by Pauling as a measure of this atomic size . tolerance of ±0 .4 Å of the sum rcov (A) + rcov (B) . The covalent radii Thus in a closely packed structure two non-bonded atoms A and are given in the fourth column of the table . B will be separated by the sum of their van der Waals radii rvdw (A) and rvdw (B) . The set of van der Waals radii proposed by Pauling References was refined by Bondi (Reference 1) based on crystallographic data, gas kinetic collision cross sections, and liquid state properties . The 1 . Bondi, A ., J. Phys. Chem. 68, 441, 1964 . non-bonded contact distances predicted from the recommended 2 . Rowland, R . S . and Taylor, R ., J. Phys. Chem. 100, 7384, 1996 . 3 . Cambridge Crystallographic Data Center, www .ccdc .cam .ac .uk/prod- r of Bondi have been compared with actual data in the collec- vdw ucts/csd/radii/ tion of the Cambridge Crystallographic Data Center by Rowland and Taylor (Reference 2) and modified slightly . -

1.1 Effective Nuclear Charge 1.2 Effective Nuclear Charge 1.3
Periodic Trends Periodic Trends 1.1 Effective Nuclear Charge 1.2 Effective Nuclear Charge The interaction between the nuclear charge and the Zeff = nuclear charge actually experienced by an valence electrons (how many? how far away?) is electron critical Simplest approximation The nuclear charge experienced by the valence Zeff = Z - # core electrons electrons (Zeff ) impacts how tightly the valence electrons are held Assumption How tightly the valence electrons are held influences atomic size, ionization energy, electron affinity, and Examples reactivity Periodic Trends Periodic Trends 1.3 Effective Nuclear Charge 1.4 Slater’s Rules Slater’s rules acknowledge the imperfect shielding Slater’s rules assume imperfect shielding caused by orbital penetration Zeff = Z – where is calculated using Slater’s rules 1. GthbitlidGroup the orbitals in order: (1s) (2s,2p) (3s,3p) (3d) (4s,4p) (4d) (4f) (5s,5p)… 2. To determine , sum up the following contributions for the electron of interest: a. 0 (zero) for all electrons in groups outside (to the right of) the one being considered b. 0.35 for each of the other electrons in the same ggp(proup (except for 1s group where 0.30 is used) c. If the electron is in a (ns,np) group, 0.85 for each electron in the next innermost (to the left) group d. If the electron is in a (nd) or (nf) group, 1.00 for each electron in the next innermost (to the left) group e. 1.00 for each electron in the still lower (farther in) groups 1 Periodic Trends Periodic Trends 1.5 Using Slater’s Rules 1.6 Using Slater’s Rules What do the 1.0, 0.85 and 0.35 factors mean? Fluorine’s Zeff calculated using the simple approximation = 7 and using Slater’s rules = 5.20. -

CHEM 1A03 UNIT 3: Periodic Trends Introduction
CHEM 1A03 UNIT 3: Periodic Trends Introduction Hey! Thanks for opening up this Chemistry Periodic Trends Review! The education team at WebStraw McMaster has put together a comprehensive breakdown for you that covers all the key concepts for Chemistry Periodic Trends Members of our team have taken the course in previous years, and we understand better than anyone else what specific ideas and concepts tend to trip students up throughout the semester. We are essentially offering you the key takeaways from the course, after having completed the course ourselves. Before you read further, also keep in mind that these review packages are not meant to be a tool for you to learn the course from scratch. The content presented below was designed with the assumption that you already have a preliminary understanding of Periodic Trends. Our goal is to help give you a more in-depth understanding of key outcomes, as well as to help you see how concepts relate/connect to one another within the scope of the course as a whole. We do our best to cover every topic within the unit; however, some testable outcomes may not be discussed. With that said, best of luck in your studying! Remember to make good use of your time, but to also take breaks as well. Yousef Abumustafa & Julia Ma The WebStraw McMaster Chemistry team WebStraw McMaster Periodic properties of elements The periodic table is divided into called columns called groups that group elements with similar chemical/physical properties together and rows called periods that group elements with the same -
![Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N](https://docslib.b-cdn.net/cover/8398/atomic-and-ionic-radii-of-elements-1-96-martinrahm-a-roald-hoffmann-a-and-n-668398.webp)
Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N
DOI:10.1002/chem.201602949 Full Paper & Elemental Radii Atomic and Ionic Radii of Elements 1–96 MartinRahm,*[a] Roald Hoffmann,*[a] and N. W. Ashcroft[b] Abstract: Atomic and cationic radii have been calculated for tive measureofthe sizes of non-interacting atoms, common- the first 96 elements, together with selected anionicradii. ly invoked in the rationalization of chemicalbonding, struc- The metric adopted is the average distance from the nucleus ture, and different properties. Remarkably,the atomic radii where the electron density falls to 0.001 electrons per bohr3, as defined in this way correlate well with van der Waals radii following earlier work by Boyd. Our radii are derived using derived from crystal structures. Arationalizationfor trends relativistic all-electron density functional theory calculations, and exceptionsinthose correlations is provided. close to the basis set limit. They offer asystematic quantita- Introduction cule,[2] but we prefer to follow through with aconsistent pic- ture, one of gauging the density in the atomic groundstate. What is the size of an atom or an ion?This question has been The attractivenessofdefining radii from the electron density anatural one to ask over the centurythat we have had good is that a) the electron density is, at least in principle, an experi- experimental metricinformation on atoms in every form of mental observable,and b) it is the electron density at the out- matter,and (more recently) reliable theory for thesesame ermost regionsofasystem that determines Pauli/exchange/ atoms. And the momentone asks this question one knows same-spinrepulsions, or attractive bondinginteractions, with that there is no unique answer.Anatom or ion coursing down achemical surrounding. -

ARC: an Open-Source Library for Calculating Properties of Alkali Rydberg Atoms
ARC: An open-source library for calculating properties of alkali Rydberg atoms N. Šibalic´a,∗, J. D. Pritchardb, C. S. Adamsa, K. J. Weatherilla aJoint Quantum Center (JQC) Durham-Newcastle, Department of Physics, Durham University, South Road, Durham, DH1 3LE, United Kingdom bDepartment of Physics, SUPA, University of Strathclyde, 107 Rottenrow East, Glasgow, G4 0NG, United Kingdom Abstract We present an object-oriented Python library for computation of properties of highly-excited Rydberg states of alkali atoms. These include single-body effects such as dipole matrix elements, excited-state lifetimes (radiative and black-body limited) and Stark maps of atoms in external electric fields, as well as two-atom interaction potentials accounting for dipole and quadrupole coupling effects valid at both long and short range for arbitrary placement of the atomic dipoles. The package is cross-referenced to precise measurements of atomic energy levels and features extensive documentation to facilitate rapid upgrade or expansion by users. This library has direct application in the field of quantum information and quantum optics which exploit the strong Rydberg dipolar interactions for two-qubit gates, robust atom-light interfaces and simulating quantum many-body physics, as well as the field of metrology using Rydberg atoms as precise microwave electrometers. Keywords: Alkali atom, Matrix elements, Dipole-dipole interactions, Stark shift, Förster resonances PROGRAM SUMMARY They are a flourishing field for quantum information process- Program Title: ARC: Alkali Rydberg Calculator ing [1, 2] and quantum optics [3, 4, 5] in the few to single exci- Licensing provisions: BSD-3-Clause tation regime, as well as many-body physics [6, 7, 8, 9, 10, 11], Programming language: Python 2.7 or 3.5, with C extension in the many-excitations limit. -

Important Factoids in Understanding Effective Nuclear Charge
CHEM 1B Important Factoids in Understanding Effective Nuclear Charge To understand the concept of effective nuclear charge, we focus on one electron (usually a valence or outer electron) and consider the forces the other charged particles exert on this electron. In an atom with more than one electron, each electron will ‘feel’ both attractive forces from the positive nucleus and repulsive forces from the other electrons. Qualitatively, Zeff is the NET (or effective) ATTRACTIVE charge that the electron ‘feels’, taking into account both the attractive and repulsive forces. The attractive part of Zeff is just Z, the positive charge on the nucleus ‘pulling’ on the electron (i.e. the atomic number) The repulsive part, repulsion from the other electrons, is referred to as ‘shielding’. It gets this designation because an electron is ‘shielded’ from the pull of the positive nucleus by the repulsion of the other electrons. To provide shielding an electron must have its probability density closer to the nucleus than the outer electron (and thus partially neutralize the + nuclear charge). Thus Zeffective=+Z ‐(shielding of other electrons) [note the + sign for the attractive part and –sign for the repulsive part] MORE SHIELDING ï LOWER Zeff LESS SHIELDING ï HIGHER Zeff In terms of Zeff and the principal quantum number n of the outer electron, the ionization energy and size of an atom can be approximated as: 22 2 18ZZeff 18 eff 11 n EnZeff, 2.18 10 J22 and IE 2.18 10 J and ravg 5.28 10 m nn Zeff An approximate Zeff can be obtained empirically from experimental ionization energies: 12 nIE2 experimental Zeff 18 2.18 10 J The lecture will discuss: For 1‐electron atoms or ions [H, He+, Li2+, Be3+, etc.] there is no shielding and Zeff∫Z [Z=+1, +2, +3, +4, etc.] For He 1s2 the second electron (in the same 1s shell) provides 0.66 shielding [reduces the pull of the nucleus from Z= +2 to Zeffº1.34] For Li 1s22s each of the two 1s electrons provides 0.84 shielding for the outer 2s electron [the two reduce the pull of the nucleus from Z=+3 to Zeffº3‐2ä0.87=1.26. -

Structures of Cobalt, Zinc and Lead Niobates Based On
1 Structures at the Atomic Level of Cobalt, Zinc and Lead Niobates Raji Heyrovska Institute of Biophysics, Academy of Sciences of the Czech Republic, 135 Kralovopolska, 612 65 Brno, Czech Republic. Email: [email protected] The author has found in recent years that bond lengths are exact sums of the radii of adjacent atoms and or ions, where the ions have Golden ratio based radii. This work was prompted by the exciting observation last year of the Golden ratio in the magnetic properties of cobalt niobate. It is shown here that in cobalt and zinc niobates, cobalt, zinc and oxygen ions have Golden ratio based ionic radii, whereas in lead niobate, all atoms have covalent radii. Also, the angles at the single bond oxygen anion and atom are close to 1080, as in a pentagon. 1-3 The experimental finding of the E8 symmetry in the magnetic properties of cobalt niobate, CoNb2O6 provoked the author's interest to look into the atomic Nature Precedings : hdl:10101/npre.2011.6059.1 Posted 24 Jun 2011 structures of niobates. It was found4-7 in recent years that the Golden sections of the covalent bond lengths d(AA) between two atoms of the same kind are sums of the radii of Pauling's ionic resonance forms8, which are the cations and anions of the atom (A), i.e., d(AA) = d(AA)/φ + d(AA)/φ2, where φ (= 51/2 + 1)/2 is the Golden ratio. In particular, the inter-ionic distances in all alkali halides (M+X-) were shown to be exact sums of the Golden ratio based ionic radii, d(M+) = d(MM)/φ2 and d(X-) = d(XX)/φ. -

AP Chemistry: 2017-18 Semester Review: Chapters 6 Thru 9, 11.2-11.3
AP Chemistry: 2017-18 Semester Review: MULTIPLE CHOICE Section Chapters 6 thru 9, 11.2-11.3 Chp. 6 1) Which one of the following is correct? A) ν ÷ λ = c B) ν = cλ C) λ = c ν D) νλ = c E) ν + λ = c Page Ref: Sec. 6.1 2) In the Bohr model of the atom, _____. A) electrons travel in circular paths called orbitals B) electron paths are controlled by probability C) electrons can have any energy D) electron energies are quantized E) both A and C Page Ref: Sec. 6.3 3) Which one of the following is an incorrect orbital notation? A) 4dxy B) 3py C) 4s D) 2s E) 3f Page Ref: Sec. 6.5 4) Which electron configuration represents a violation of the Pauli exclusion principle? A) B) C) D) E) Page Ref: Sec. 6.8 1 5) The ground state electron configuration of Ga is __________. A) B) C) D) E) [Ar] Page Ref: Sec. 6.8 6) Which electron configuration represents a violation of Hund's rule for an atom in its ground state? A) B) C) D) E) Page Ref: Sec. 6.8 7) Which two elements have the same ground-state electron configuration? A) Pd and Pt B) Fe and Cu C) Cu and Ag D) Cl and Ar E) No two elements have the same ground-state electron configuration. Page Ref: Sec. 6.8 Chp. 7 1) In which set of elements would all members be expected to have very similar chemical properties? A) O, S, Se B) S, Se, Si C) N, O, F D) Ne, Na, Mg E) Na, Mg, K Page Ref: Sec. -

Chalcogen-Nitrogen Bond: Insights Into a Key Chemical Motif
Proceedings Chalcogen-nitrogen Bond: Insights into A Key Chemical Motif Marco Bortoli,1 Andrea Madabeni,1 Pablo Andrei Nogara,2 Folorunsho B. Omage,2 Giovanni Ribaudo,3 Davide Zeppilli,1 Joao Batista Teixeira Rocha,2* Laura Orian1* 1 Dipartimento di Scienze Chimiche Università degli Studi di Padova Via Marzolo 1 35131 Padova, Italy; [email protected] (M.B.); [email protected] (A.M..); [email protected] (D.Z.) 2 Departamento de Bioquímica e Biologia Molecular, Universidade Federal de Santa Maria, Santa Maria, 97105-900, RS Brazil; [email protected] (P.A.N.); [email protected] (F.B.O.) 3 Dipartimento di Medicina Molecolare e Traslazionale, Università degli Studi di Brescia, Viale Europa 11, 25123 Brescia, Italy; [email protected] (G.R.) * Correspondence: : [email protected] (J.B.T.R), [email protected] (L.O.); † Presented at the 1st International Electronic Conference on Catalysis Sciences, 10–30 November 2020; Available online: https://eccs2020.sciforum.net/ Published: 10 November 2020 Abstract: Chalcogen-nitrogen chemistry deals with systems in which sulfur, selenium or tellurium is linked to a nitrogen nucleus. This chemical motif is a key component of different functional structures, ranging from inorganic materials and polymers to rationally designed catalysts, to bioinspired molecules and enzymes. The formation of a selenium-nitrogen bond, and its disruption, are rather common events in organic Se-catalyzed processes. In nature, along the mechanistic path of glutathione peroxidase, evidence of the formation of a Se-N bond in highly oxidizing conditions has been reported and interpreted as a strategy to protect the selenoenzyme from overoxidation. -

Lanthanides.Pdf
Lanthanides [A] LANTHANIDES : 4f block elements Definition: The f- block (inner transition) elements containing partially filled 4f-subshells are known as Lanthanides or Lanthanones because of their close similarities with element lanthanum (atomic no: 57). The fourteen elements from atomic no: 58 to 71 constitute lanthanides. Nos. Name Symbol Electronic configuration 0 1 2 1. Lanthanum La57 [Xe] 4f 5d 6s 2 0 2 2. Cerium Ce58 [Xe] 4f 5d 6s 3 0 2 3. Praseodymium Pr59 [Xe] 4f 5d 6s 4 0 2 4. Neodymium Nd60 [Xe] 4f 5d 6s 5 0 2 5. Promethium Pm61 [Xe]4f 5d 6s 6 0 2 6. Samarium Sm62 [Xe]4f 5d 6s 7 0 2 7. Europium Eu63 [Xe] 4f 5d 6s 7 1 2 8. Gadolinium Gd64 [Xe] 4f 5d 6s 9 0 2 9. Terbium Tb65 [Xe] 4f 5d 6s 10 0 2 10. Dysprosium Dy66 [Xe] 4f 5d 6s 11 0 2 11. Holmium Ho67 [Xe] 4f 5d 6s 12 0 2 12. Erbium Er68 [Xe] 4f 5d 6s 13 0 2 13. Thulium Tm69 [Xe] 4f 5d 6s 14 0 2 14. Ytterbium Yb70 [Xe] 4f 5d 6s 14 1 2 15. Lutetium Lu71 [Xe] 4f 5d 6s From the above electronic configuration it can be seen that at La 5d orbital is singly occupied but after La further filling of 5d orbital is discontinued. As the nuclear charge increases by one unit from La to Ce, 4f orbitals were higher in energy upto Lu, fall slightly below the 5d level 4f- orbitals, therefore begin to fill and are completely filled up to Lu, before filling of 5d orbital is resumed. -

Van Der Waals Radii of Elements S
Inorganic Materials, Vol. 37, No. 9, 2001, pp. 871–885. Translated from Neorganicheskie Materialy, Vol. 37, No. 9, 2001, pp. 1031–1046. Original Russian Text Copyright © 2001 by Batsanov. Van der Waals Radii of Elements S. S. Batsanov Center for High Dynamic Pressures, Mendeleevo, Solnechnogorskii raion, Moscow oblast, 141570 Russia Received February 14, 2001 Abstract—The available data on the van der Waals radii of atoms in molecules and crystals are summarized. The nature of the continuous variation in interatomic distances from van der Waals to covalent values and the mechanisms of transformations between these types of chemical bonding are discussed. INTRODUCTION der Waals radius with the quantum-mechanical require- ment that the electron density vary continuously at the The notion that an interatomic distance can be periphery of atoms. thought of as the sum of atomic radii was among the most important generalizations in structural chemistry, In this review, the van der Waals radii of atoms eval- treating crystals and molecules as systems of interact- uated from XRD data, molar volumes, physical proper- ing atoms (Bragg, 1920). The next step forward in this ties, and crystal-chemical considerations are used to area was taken by Mack [1] and Magat [2], who intro- develop a universal system of van der Waals radii. duced the concept of nonvalent radius (R) for an atom situated at the periphery of a molecule and called it the atomic domain radius [1] or Wirkungsradius [2], ISOTROPIC CRYSTALLOGRAPHIC implying that this radius determines intermolecular dis- VAN DER WAALS RADII tances. Later, Pauling [3] proposed to call it the van der Kitaigorodskii [4, 5] was the first to formulate the Waals radius, because it characterizes van der Waals principle of close packing of molecules in crystalline interactions between atoms. -

Chapter 7 Periodic Properties of the Elements Learning Outcomes
Chapter 7 Periodic Properties of the Elements Learning Outcomes: Explain the meaning of effective nuclear charge, Zeff, and how Zeff depends on nuclear charge and electron configuration. Predict the trends in atomic radii, ionic radii, ionization energy, and electron affinity by using the periodic table. Explain how the radius of an atom changes upon losing electrons to form a cation or gaining electrons to form an anion. Write the electron configurations of ions. Explain how the ionization energy changes as we remove successive electrons, and the jump in ionization energy that occurs when the ionization corresponds to removing a core electron. Explain how irregularities in the periodic trends for electron affinity can be related to electron configuration. Explain the differences in chemical and physical properties of metals and nonmetals, including the basicity of metal oxides and the acidity of nonmetal oxides. Correlate atomic properties, such as ionization energy, with electron configuration, and explain how these relate to the chemical reactivity and physical properties of the alkali and alkaline earth metals (groups 1A and 2A). Write balanced equations for the reactions of the group 1A and 2A metals with water, oxygen, hydrogen, and the halogens. List and explain the unique characteristics of hydrogen. Correlate the atomic properties (such as ionization energy, electron configuration, and electron affinity) of group 6A, 7A, and 8A elements with their chemical reactivity and physical properties. Development of Periodic Table •Dmitri Mendeleev and Lothar Meyer (~1869) independently came to the same conclusion about how elements should be grouped in the periodic table. •Henry Moseley (1913) developed the concept of atomic numbers (the number of protons in the nucleus of an atom) 1 Predictions and the Periodic Table Mendeleev, for instance, predicted the discovery of germanium (which he called eka-silicon) as an element with an atomic weight between that of zinc and arsenic, but with chemical properties similar to those of silicon.