PARIS FOLDAMERS 2013 SYMPOSIUM 10-12 April 2013 Short Lecture SL1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monday, 15 June 2009 Tuesday, 16 June 2009
Monday, 15 June 2009 8:00-17:00 Registration (Lobby, ground floor) 9:00-18:30 PARALLEL WORKSHOPS - AOC 2009 (Ialysos, 1st floor) (http://cnd.iit.cnr.it/aoc2009/) - HotMESH 2009 (Asclipios, 1st floor) (http://cnd.iit.cnr.it/mesh09/) - IREHSS 2009 (Delphi, 1st floor) (http://www.irehss.org/) Tuesday, 16 June 2009 8:00-17:00 Registration (Lobby, ground floor) 8:30-09:00 WELCOME ADDRESS (Colossos, ground floor) 9:00-10:30 Session 1: Best paper candidates (Colossos, ground floor) (Session chairs: Prasant Mohapatra and Jörg Ott) A Joint Routing and Scheduling Scheme for Wireless Networks with Multi-packet Reception and Directional Antennas Jorge Crichigno (University of New Mexico, US) Admission Control based on OFDMA Channel Transformations James Gross (RWTH Aachen University, DE) A CDMA-Based Approach for Highly Efficient Medium Access Control in Mesh Wireless Networks Su Xia , Hongyi Wu (University of Louisiana at Lafayette, US) 10:30-11:00 Coffee break (Lobby, ground floor) 11:00-12:00 Keynote Speech I (Colossos, ground floor): Cooperation at the Network Level Anthony Ephremides, University of Maryland, USA 12:00-13:30 Lunch (1st floor) 13:30-15:15 Session 2a: MAC Protocols (Colossos, ground floor) (Session chair: Vania Conan, Thales) Multi-Channel Spectrum-Agile MAC Protocol with Adaptive Load Control Fan Wang, Marwan Krunz (University of Arizona, US) MAC FER-Based Codec Adaptation for Multimedia Streaming over Wireless Networks Chun-Cheng Chiang, Zhung-Han Wu, Cheng-Yu Shih, Ping-Cheng Yeh, Hung-Yun Hsieh (National Taiwan University, TW) -

Listado De Instituciones De Educación Superior Extranjeras Cuyos Títulos
TABLA 1.- Listado de Instituciones de Educación Superior Extranjeras cuyos títulos han sido reconocidos por SENESCYT FUENTE: Sistema Nacional de Información de la Educación Superior - SNIESE ELABORADO POR: Dirección Nacional de Gestión de la Información - DNGI NOTAS TÉCNICAS: * La infomación tiene fecha corte 17 de diciembre de 2020. Nro. -

Owning the Right to Open up Access to Scientific Publications Guibault, L
UvA-DARE (Digital Academic Repository) Owning the right to open up access to scientific publications Guibault, L. Published in: Open content licensing: from theory to practice DOI: 10.5117/9789089643070 Link to publication Citation for published version (APA): Guibault, L. (2011). Owning the right to open up access to scientific publications. In L. Guibault, & C. Angelopoulos (Eds.), Open content licensing: from theory to practice (pp. 137-167). Amsterdam: Amsterdam University Press. DOI: 10.5117/9789089643070 General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: http://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (http://dare.uva.nl) Download date: 15 Dec 2018 Open Content Licensing From Theory to Practice Edited by Lucie Guibault & Christina Angelopoulos Amsterdam University Press Cover design: Kok Korpershoek bno, Amsterdam Lay-out: JAPES, Amsterdam ISBN 978 90 8964 307 0 e-ISBN 978 90 4851 408 3 NUR 820 Creative Commons License CC BY NC (http://creativecommons.org/licenses/by-nc/3.0) L. -

Paul Weirich Curriculum Vitae August, 2020
Paul Weirich Curriculum Vitae August, 2020 Curators’ Distinguished Professor, Philosophy Department University of Missouri, Columbia, MO 65211 Telephone: (573) 882-2871, Fax: (573) 884-8949 E-mail: [email protected] Education Ph.D., philosophy, UCLA, 1977. Supervisor: Tyler Burge. Dissertation: Probability and Utility for Decision Theory. Published in 1977 on microfilm by University Microfilms, Ann Arbor, MI. Published in 2010 in print by VDM, Saarbrücken, Germany. B.A., philosophy, St. Louis University, 1968. Minor in French, magna cum laude, Phi Beta Kappa, mathematics and French honor fraternities. Languages: French, some German and Latin. Employment From 2010 Curators’ Distinguished Professor, 1997–2010 Professor, and 1988–97, Associate Professor, Philosophy Department, University of Missouri. 1987–88, Assistant Professor, Philosophy Program, California State Polytechnic University, Pomona. 1980–87, Assistant Professor, Philosophy Department, University of Rochester. 1978–80, Instructor Part-Time, Philosophy Department, University of Rochester (concurrently a Mellon Postdoctoral Fellow). 1977–78, Visiting Assistant Professor, Philosophy Department, University of New Orleans. 1975, summer, Instructor Part-Time, Philosophy Department, UCLA. Courses taught. Graduate: decision theory (including game theory), logic (inductive logic, epistemic logic, the logic of conditionals, theories of truth, causal inference, deontic logic), social and political philosophy, philosophy of science (Bayesian epistemology), teaching philosophy. Undergraduate: -

Encyclopedia of Intensive Care Medicine
Encyclopedia of Intensive Care Medicine Jean-Louis Vincent and Jesse B. Hall (Eds) Encyclopedia of Intensive Care Medicine With 716 Figures and 450 Tables Editors Jean-Louis Vincent Head Dept of Intensive Care Erasme Hospital (Free University of Brussels) Route de Lennik 808 1070 Brussels Belgium Jesse B. Hall University of Chicago Medical Center 5841 S. Maryland Ave. MC 6076 Chicago, IL 60637 USA ISBN 978-3-642-00417-9 e-ISBN 978-3-642-00418-6 Print and electronic bundle under ISBN 978-3-642-00419-3 DOI 10.1007/978-3-642-00418-6 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2012931922 © Springer-Verlag Berlin Heidelberg 2012 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Printed on acid-free paper Springer is part of Springer ScienceþBusiness Media (www.springer.com) List of Contributors N. -

Lecture Notes in Computer Science 7289 Commenced Publication in 1973 Founding and Former Series Editors: Gerhard Goos, Juris Hartmanis, and Jan Van Leeuwen
Lecture Notes in Computer Science 7289 Commenced Publication in 1973 Founding and Former Series Editors: Gerhard Goos, Juris Hartmanis, and Jan van Leeuwen Editorial Board David Hutchison Lancaster University, UK Takeo Kanade Carnegie Mellon University, Pittsburgh, PA, USA Josef Kittler University of Surrey, Guildford, UK Jon M. Kleinberg Cornell University, Ithaca, NY, USA Alfred Kobsa University of California, Irvine, CA, USA Friedemann Mattern ETH Zurich, Switzerland John C. Mitchell Stanford University, CA, USA Moni Naor Weizmann Institute of Science, Rehovot, Israel Oscar Nierstrasz University of Bern, Switzerland C. Pandu Rangan Indian Institute of Technology, Madras, India Bernhard Steffen TU Dortmund University, Germany Madhu Sudan Microsoft Research, Cambridge, MA, USA Demetri Terzopoulos University of California, Los Angeles, CA, USA Doug Tygar University of California, Berkeley, CA, USA Gerhard Weikum Max Planck Institute for Informatics, Saarbruecken, Germany Robert Bestak Lukas Kencl Li Erran Li Joerg Widmer Hao Yin (Eds.) NETWORKING 2012 11th International IFIP TC 6 Networking Conference Prague, Czech Republic, May 21-25, 2012 Proceedings, Part I 13 Volume Editors Robert Bestak Lukas Kencl Czech Technical University in Prague Department of Telecommunication Engineering Technicka 2, 166 27 Prague 6, Czech Republic E-mail: {robert.bestak, lukas.kencl}@fel.cvut.cz Li Erran Li Bell Labs, Alcatel Lucent 600 Mountain Avenue, Murray Hill, NJ 07974-0636, USA E-mail: [email protected] Joerg Widmer Institute IMDEA Networks -
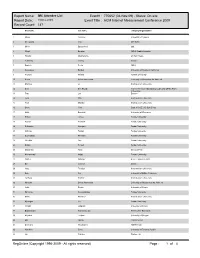
Attendee List Event# : 770652 (04-Nov-09) - Status: On-Site Report Date: 19-Nov-2009 Event Title : ACM Internet Measurement Conference 2009 Record Count: 147
Report Name: IMC Attendee List Event# : 770652 (04-Nov-09) - Status: On-site Report Date: 19-Nov-2009 Event Title : ACM Internet Measurement Conference 2009 Record Count: 147 first name last name company/organization 1 Niklas Carlsson University of Calgary 2 Meeyoung Cha MPI-SWS 3 Oliver Spatscheck at&t 4 Chadi Barakat INRIA Sophia Antipolis 5 Harsha Madhyastha UC San Diego 6 Yuchung Cheng Google 7 Daniele Perito INRIA 8 Genevieve Bartlett University of Southern California 9 Andrew Kalafut Indiana University 10 Aruna Balasubramanian University of Masachusetts Amherst 11 Zhichun Li Northwestern University 12 Zied Ben Houidi France Telecom R&D Orange Labs and UPMC Paris Universitas 13 Tom Los Endace 14 John Otto Northwestern University 15 Amit Mondal Northwestern University 16 Scott Yilek Dept. of CSE, UC San Diego 17 Holly Esquivel University of Wisconsin 18 Ruben Torres Purdue University 19 Pawan Prakash Purdue University 20 Sriharsha Gangam Purdue University. 21 Abhinav Pathak Purdue University 22 Soumyadip Banerjee Purdue University 23 Wei-Min Yao Purdue University 24 Debbie Perouli Purdue University 25 Mukarram Tariq Georgia Tech 26 Mohammad Hajjat Purdue University 27 Walter Willinger AT&T Labs-Research 28 Bill Cantrell Endace 29 Ionut Trestian Northwestern University 30 Kan Cai University of British Columbia 31 Zachary Bischof Northwestern University 32 Niranjan Balasubramanian University of Massachusetts Amherst 33 Ankit Singla University of Illinois 34 Dimitrios Koutsonikolas Purdue University 35 Mario Sanchez Northwestern University -

Février 2009 PROJET « UNIVERSITE PARIS CITE »
Paris, Ville Universitaire OPERATION CAMPUS Février 2009 PROJET « UNIVERSITE PARIS CITE » 1 2 3 S O M M A I R E PRES UNIVERSITE PARIS CITE Signatures des déposants 2 Sommaire 5 Les territoires Campus parisiens 7 Sites et disciplines du Campus 9 I. Introduction 11 I.1 Pourquoi un PRES 11 I.2 Quelle stratégie d’ensemble ? 12 I.3. Quels projets communs ? 13 II. Présentation du projet 15 II.1. Les partenaires 15 II.2. Les territoires Campus 22 II.3. La gouvernance : Projets de statuts du PRES 27 III. Les projets pédagogiques et scientifiques du PRES 33 III.1. Biologie – Médecine 33 III.2. Santé et société 36 III.3 Le Centre de Santé Publique de l’Hôtel Dieu de Paris 38 III.4 Langues et civilisations 39 III.5. La formation technologique 39 III.6 Autres projets de formations communes 40 IV. Les projets transversaux coopératifs 47 IV.1. Développement de stratégies communes de valorisation du PRES 47 IV.2. Politique et promotion internationale du PRES 48 IV.3. Mutualisation des fonctions supports 50 IV.4. Valorisation du patrimoine immobilier 51 IV.5. Systèmes d’information et TICE 52 IV.6. Bibliothèques – PRES – Université Paris Cité 54 V. Vie et Santé étudiante 55 V.I. Logements et installations sportives 55 V.2. La santé étudiante 55 VI. Conclusion 57 VII. Annexes 59 VII.I. Projets vie étudiante (Paris Ville Universitaire) 59 VII.2. Projets propres au PRES 65 VII.3 Récapitulatif des projets immobiliers propres au PRES 67 VII.4. Récapitulatif des projets immobiliers communes Paris Ville Universitaire, opérations 69 d’intérêt pour le PRES « Université Paris Cité ». -
Final Program Page 2 SAC 2010, March 21 – 26
APPLIED COMPUTING 2010 The 25th Annual ACM Symposium on Applied Computing PROCEEDINGS OF THE 2010 ACM SYMPOSIUM ON APPLIED COMPUTING Sierre, Switzerland March 22-26, 2010 Organizing Committee Sung Y. Shin Dongwan Shin Sascha Ossowski Boi Faltings Michael Schumacher Jiman Hong Mathew J. Palakal Christelle Monnet Chih-Cheng Hung Hisham M. Haddad Hosted by University of Applied Sciences Western Switzerland (HES-SO) and Ecole Polytechnique Fédérale de Lausanne (EPFL) Barrett R. Bryant, Steering Committee Member University of Alabama at Birmingham, USA SAC 2010 Roger L. Wainwright, Steering Committee Member University of Tulsa, USA Track Chairs Introduction Volume I: Distributed Systems & SAC 2010 is a premier international conference on Engineering applied computing and technology. Attendees have the opportunity to hear from expert practitioners and Document Engineering (DE) researchers about the latest trends in research and Rafael Lins, Universidade Federal de Pernambuco, development in their fields. SAC 2010 features two Brazil keynote speakers on Tuesday and Thursday, from Enterprise Engineering (EE) 9:00 to 10:40. The technical program of the Artur Caetano, IST, Technical University of Lisbon, symposium consists of 43 tracks on different research Portugal topics, which run from Tuesday March 23 through Rogério Carvalho, IFF, Brazil Friday March 26, 2010. Regular oral presentation Maria-Eugenia Iacob, University of Twente, sessions start at 9:00 and end at 18:00 in six parallel Netherlands sessions. In addition, two poster tracks also run on Martin Op’t Land, Capgemini and Delft University of Wednesday March 24, from 9:40 to 12:40 and from Technology, Netherlands 14:40 to 17:40. -

Political Theory
Political Theory http://ptx.sagepub.com/ On Political Liberty: Montesquieu's Missing Manuscript Annelien de Dijn Political Theory 2011 39: 181 DOI: 10.1177/0090591710394088 The online version of this article can be found at: http://ptx.sagepub.com/content/39/2/181 Published by: http://www.sagepublications.com Additional services and information for Political Theory can be found at: Email Alerts: http://ptx.sagepub.com/cgi/alerts Subscriptions: http://ptx.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav Citations: http://ptx.sagepub.com/content/39/2/181.refs.html Downloaded from ptx.sagepub.com at UNIV OF NOTRE DAME on April 9, 2011 Special Section: Enlightenments Political Theory 39(2) 181 –204 On Political Liberty: © 2011 SAGE Publications Reprints and permission: http://www. Montesquieu’s Missing sagepub.com/journalsPermissions.nav DOI: 10.1177/0090591710394088 Manuscript http://ptx.sagepub.com Annelien de Dijn1 Abstract This essay draws attention to the importance of Montesquieu’s earliest and unpublished writings on liberty for our understanding of the famous eleventh book of the Spirit of the Laws. Montesquieu’s investigation of the nature and preconditions of liberty, the author argues, was much more polemical than it is usually assumed. As an analysis of his notebooks shows, Montesquieu set out to wrest control over the concept of liberty from the republican admirers of classical antiquity, a faction that he believed to be dangerously populist and revolutionary. In order to do so, Montesquieu came up with a redefinition of the concept of liberty that allowed him to argue that monar- chical subjects could be just as free as republican citizens. -

Complementary Roles of the Rat Prefrontal Cortex and Striatum in Reward-Based Learning and Shifting Navigation Strategies Mehdi Khamassi
Complementary roles of the rat prefrontal cortex and striatum in reward-based learning and shifting navigation strategies Mehdi Khamassi To cite this version: Mehdi Khamassi. Complementary roles of the rat prefrontal cortex and striatum in reward-based learning and shifting navigation strategies. Cognitive Sciences. Université Pierre et Marie Curie - Paris VI, 2007. English. tel-00688927 HAL Id: tel-00688927 https://tel.archives-ouvertes.fr/tel-00688927 Submitted on 18 Apr 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Complementary roles of the rat prefrontal cortex and striatum in reward-based learning and shifting navigation strategies: Electrophysiological and computational studies, application to simulated autonomous robotics. Mehdi KHAMASSI PhD thesis – Université Pierre et Marie Curie (Paris Universitas) 2007 Speciality COGNITIVE SCIENCE Presented on september 26th 2007, in presence of the jury: Pr. Alain Berthoz Examinator LPPA, Collège de France Pr. Philippe Bidaud President of the jury ISIR, Université Paris 6 Dr. Kenji Doya Reviewer IRP, Okinawa Institute of Sci. and Tech. Dr. Agnès Guillot Thesis director LIP6/ISIR, Université Paris X Pr. Cyriel Pennartz Examinator SILS, Universiteit van Amsterdam Dr. Bruno Poucet Reviewer LNC, CNRSUniversité de Provence Dr. -
Uva-DARE (Digital Academic Repository)
UvA-DARE (Digital Academic Repository) Owning the right to open up access to scientific publications Guibault, L. DOI 10.5117/9789089643070 Publication date 2011 Document Version Final published version Published in Open content licensing: from theory to practice Link to publication Citation for published version (APA): Guibault, L. (2011). Owning the right to open up access to scientific publications. In L. Guibault, & C. Angelopoulos (Eds.), Open content licensing: from theory to practice (pp. 137- 167). Amsterdam University Press. https://doi.org/10.5117/9789089643070 General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:01 Oct 2021 Open Content Licensing From Theory to Practice Edited by Lucie Guibault & Christina Angelopoulos Amsterdam University Press Cover design: Kok Korpershoek bno, Amsterdam Lay-out: JAPES, Amsterdam ISBN 978 90 8964 307 0 e-ISBN 978 90 4851 408 3 NUR 820 Creative Commons License CC BY NC (http://creativecommons.org/licenses/by-nc/3.0) L.