University of Florida Thesis Or Dissertation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plumage Coloration and Morphology in Chiroxiphia Manakins
PLUMAGE COLORATION AND MORPHOLOGY IN CHIROXIPHIA MANAKINS: INTERACTING EFFECTS OF NATURAL AND SEXUAL SELECTION Except where reference is made to the work of others, the work described in this dissertation is my own or was done in collaboration with my advisory committee. This dissertation does not include proprietary or classified information. _________________________________________ Stéphanie M. Doucet Certificate of Approval: _____________________ _____________________ F. Stephen Dobson Geoffrey E. Hill, Chair Professor Schamagel Professor Biological Sciences Biological Sciences ______________________ ______________________ Craig Guyer Stephen L. McFarland Professor Acting Dean Biological Sciences Graduate School PLUMAGE COLORATION AND MORPHOLOGY IN CHIROXIPHIA MANAKINS: INTERACTING EFFECTS OF NATURAL AND SEXUAL SELECTION Stéphanie M. Doucet A Dissertation Submitted to the Graduate Faculty of Auburn University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Auburn, Alabama May 11, 2006 PLUMAGE COLORATION AND MORPHOLOGY IN CHIROXIPHIA MANAKINS: INTERACTING EFFECTS OF NATURAL AND SEXUAL SELECTION Stéphanie M. Doucet Permission is granted to Auburn University to make copies of this dissertation at its discretion, upon request of individuals or institutions and at their expense. The author reserves all publication rights. ____________________________________ Signature of Author ____________________________________ Date of Graduation iii DISSERTATION ABSTRACT PLUMAGE COLORATION AND MORPHOLOGY IN CHIROXIPHIA MANAKINS: INTERACTING EFFECTS OF NATURAL AND SEXUAL SELECTION Stéphanie M. Doucet Doctor of Philosophy, May 11, 2006 (M.S. Queen’s University, 2002) (B.S. Queen’s University, 2000) 231 Typed Pages Directed by Dr. Geoffrey E. Hill I examined how natural and sexual selection may have influenced the morphology and coloration of Chiroxiphia manakins (Aves: Pipridae). In the first chapter, I investigated age– and sex–related patterns of plumage coloration and molt timing in long–tailed manakins, C. -

Troglodytidae Species Tree
Troglodytidae I Rock Wren, Salpinctes obsoletus Canyon Wren, Catherpes mexicanus Sumichrast’s Wren, Hylorchilus sumichrasti Nava’s Wren, Hylorchilus navai Salpinctinae Nightingale Wren / Northern Nightingale-Wren, Microcerculus philomela Scaly-breasted Wren / Southern Nightingale-Wren, Microcerculus marginatus Flutist Wren, Microcerculus ustulatus Wing-banded Wren, Microcerculus bambla ?Gray-mantled Wren, Odontorchilus branickii Odontorchilinae Tooth-billed Wren, Odontorchilus cinereus Bewick’s Wren, Thryomanes bewickii Carolina Wren, Thryothorus ludovicianus Thrush-like Wren, Campylorhynchus turdinus Stripe-backed Wren, Campylorhynchus nuchalis Band-backed Wren, Campylorhynchus zonatus Gray-barred Wren, Campylorhynchus megalopterus White-headed Wren, Campylorhynchus albobrunneus Fasciated Wren, Campylorhynchus fasciatus Cactus Wren, Campylorhynchus brunneicapillus Yucatan Wren, Campylorhynchus yucatanicus Giant Wren, Campylorhynchus chiapensis Bicolored Wren, Campylorhynchus griseus Boucard’s Wren, Campylorhynchus jocosus Spotted Wren, Campylorhynchus gularis Rufous-backed Wren, Campylorhynchus capistratus Sclater’s Wren, Campylorhynchus humilis Rufous-naped Wren, Campylorhynchus rufinucha Pacific Wren, Nannus pacificus Winter Wren, Nannus hiemalis Eurasian Wren, Nannus troglodytes Zapata Wren, Ferminia cerverai Marsh Wren, Cistothorus palustris Sedge Wren, Cistothorus platensis ?Merida Wren, Cistothorus meridae ?Apolinar’s Wren, Cistothorus apolinari Timberline Wren, Thryorchilus browni Tepui Wren, Troglodytes rufulus Troglo dytinae Ochraceous -

MS0803 Freeman & Greeney
82 Ornitología Colombiana No.7 (2008):82-85 FIRST DESCRIPTION OF THE NEST, EGGS, AND COOPERATIVE BREEDING BEHAVIOR IN SHARPE’S WREN ( CINNYCERTHIA OLIVASCENS ) Primera descripción del nido, los huevos y comportamiento de cría cooperativa en el Soterrey Caferrojizo ( Cynnicerthia olivascens ) Ben G. Freeman & Harold F. Greeney Yanayacu Biological Station and Center for Creative Studies c/o Foch 721 y Amazonas, Quito, Ecuador. e-mail: [email protected] ABSTRACT We provide the first description of the nest, eggs and nestlings of Sharpe’s Wren ( Cinnycerthia olivascens ) from northeastern Ecuador, and we document the occurrence of cooperative breeding in this species. Cinnycerthia olivascens builds large enclosed ball nests with a downward-projecting tubular entrance. The eggs are off-white with sparse reddish-brown speckling. We show that at least three adults may participate in nest-building and probably contribute to nestling provisioning. Our video observations document fledgling and show that the nestlings’ diet consisted of small invertebrates. Key words: Cinnycerthia olivascens , cooperative breeding, natural history, Sharpe’s Wren . RESUMEN Describimos por la primera vez el nido, los huevos y los pichones del Soterrey Caferrojizo (Cinnycerthia olivascens ) del noreste de Ecuador, y documentamos la existencia de cría cooperativa en esta especie. Cinnycerthia olivascens construye un nido encerrado, en forma de bola, con una entrada tubular. Los huevos son blancuzcos con manchas de color canela. Por lo menos tres adultos construyen el nido, y probablemente contribuyen al cuidado de los pichones. Por medio de grabaciones de video se documentó la salida de los pichones del nido y se observó que su dieta de los pichones consiste de invertebrados pequeños. -

The Social System of Sharpe's Wren (Cinnycerthia Olivascens)
The social system of Sharpe’s Wren (Cinnycerthia olivascens): Artículo fluid group composition in a cooperative breeder El sistema social del cucarachero de Sharpe: fluidez en la composición de grupos en un ave con cría cooperativa. Gustavo H. Kattan1,2 Mónica Parada2 & J. William Beltrán2,3 1 Departamento de Ciencias Naturales y Matemáticas, Pontificia Universidad Javeriana Cali, Calle 18 No. 118-250, Cali, Colombia. 2 Ornitología Colombiana Ornitología Fundación EcoAndina, Carrera 2 A Oeste No. 12-111, Cali, Colombia. 3 Department of Biology, University of Puerto Rico, San Juan PR 00931 [email protected] Abstract Cooperatively breeding birds live in groups that normally originate in the retention of offspring, which delay dispersal and stay in the parental territory, helping to raise close kin. Group transfers usually occur when individuals disperse to obtain re- productive positions. Between 1995 and 2000 we studied the social system of Sharpe's Wren (Cinnycerthia olivascens) in an Andean forest. Wrens lived all the time in groups of up to seven individuals that maintained year-round, all-purpose territo- ries (mean = 7.6 ha) and raised offspring cooperatively. Each group had only one breeding pair, and produced 1-3 fledglings. Group composition was highly variable. Birds of all age classes frequently joined and abandoned groups. Birds became helpers when joining new groups. In one case a female breeding vacancy was filled by a bird that had joined the group two and a half years earlier. Four focal groups each had up to 20 different, temporary members in three years of ob- servation, and only the breeding pair was permanent. -

PERU: Manu and Machu Picchu Aug-Sept
Tropical Birding Trip Report PERU: Manu and Machu Picchu Aug-Sept. 2015 A Tropical Birding SET DEPARTURE tour PERU: MANU and MACHU PICCHU th th 29 August – 16 September 2015 Tour Leader: Jose Illanes Andean Cock-of-the-rock near Cock-of-the-rock Lodge! Species highlighted in RED are the ones illustrated with photos in this report. INTRODUCTION Not everyone is fortunate enough to visit Peru; a marvelous country that boasts a huge country bird list, which is second only to Colombia. Unlike our usual set departure, we started out with a daylong extension to Lomas de Lachay first, before starting out on the usual itinerary for the main tour. On this extra day we managed to 1 www.tropicalbirding.com +1-409-515-0514 [email protected] Page Tropical Birding Trip Report PERU: Manu and Machu Picchu Aug-Sept. 2015 find many extra birds like Peruvian Thick-knee, Least Seedsnipe, Peruvian Sheartail, Raimondi’s Yellow- Finch and the localized Cactus Canastero. The first site of the main tour was Huacarpay Lake, near the beautiful Andean city of Cusco (accessed after a short flight from Lima). This gave us a few endemic species like Bearded Mountaineer and Rusty-fronted Canastero; along with other less local species like Many-colored Rush-tyrant, Plumbeous Rail, Puna Teal, Andean Negrito and Puna Ibis. The following day we birded along the road towards Manu where we picked up birds like Peruvian Sierra-Finch, Chestnut-breasted Mountain-Finch, Spot-winged Pigeon, and a beautiful Peruvian endemic in the form of Creamy-crested Spinetail. We also saw Yungas Pygmy-Owl, Black-faced Ibis, Hooded and Scarlet-bellied Mountain- Tanagers, Red-crested Cotinga and the gorgeous Grass-green Tanager. -

BIRDS of BOLIVIA UPDATED SPECIES LIST (Version 03 June 2020) Compiled By: Sebastian K
BIRDS OF BOLIVIA UPDATED SPECIES LIST (Version 03 June 2020) https://birdsofbolivia.org/ Compiled by: Sebastian K. Herzog, Scientific Director, Asociación Armonía ([email protected]) Status codes: R = residents known/expected to breed in Bolivia (includes partial migrants); (e) = endemic; NB = migrants not known or expected to breed in Bolivia; V = vagrants; H = hypothetical (observations not supported by tangible evidence); EX = extinct/extirpated; IN = introduced SACC = South American Classification Committee (http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm) Background shading = Scientific and English names that have changed since Birds of Bolivia (2016, 2019) publication and thus differ from names used in the field guide BoB Synonyms, alternative common names, taxonomic ORDER / FAMILY # Status Scientific name SACC English name SACC plate # comments, and other notes RHEIFORMES RHEIDAE 1 R 5 Rhea americana Greater Rhea 2 R 5 Rhea pennata Lesser Rhea Rhea tarapacensis , Puna Rhea (BirdLife International) TINAMIFORMES TINAMIDAE 3 R 1 Nothocercus nigrocapillus Hooded Tinamou 4 R 1 Tinamus tao Gray Tinamou 5 H, R 1 Tinamus osgoodi Black Tinamou 6 R 1 Tinamus major Great Tinamou 7 R 1 Tinamus guttatus White-throated Tinamou 8 R 1 Crypturellus cinereus Cinereous Tinamou 9 R 2 Crypturellus soui Little Tinamou 10 R 2 Crypturellus obsoletus Brown Tinamou 11 R 1 Crypturellus undulatus Undulated Tinamou 12 R 2 Crypturellus strigulosus Brazilian Tinamou 13 R 1 Crypturellus atrocapillus Black-capped Tinamou 14 R 2 Crypturellus variegatus -

Northern Peru Marañon Endemics & Marvelous Spatuletail 4Th to 25Th September 2016
Northern Peru Marañon Endemics & Marvelous Spatuletail 4th to 25th September 2016 Marañón Crescentchest by Dubi Shapiro This tour just gets better and better. This year the 7 participants, Rob and Baldomero enjoyed a bird filled trip that found 723 species of birds. We had particular success with some tricky groups, finding 12 Rails and Crakes (all but 1 being seen!), 11 Antpittas (8 seen), 90 Tanagers and allies, 71 Hummingbirds, 95 Flycatchers. We also found many of the iconic endemic species of Northern Peru, such as White-winged Guan, Peruvian Plantcutter, Marañón Crescentchest, Marvellous Spatuletail, Pale-billed Antpitta, Long-whiskered Owlet, Royal Sunangel, Koepcke’s Hermit, Ash-throated RBL Northern Peru Trip Report 2016 2 Antwren, Koepcke’s Screech Owl, Yellow-faced Parrotlet, Grey-bellied Comet and 3 species of Inca Finch. We also found more widely distributed, but always special, species like Andean Condor, King Vulture, Agami Heron and Long-tailed Potoo on what was a very successful tour. Top 10 Birds 1. Marañón Crescentchest 2. Spotted Rail 3. Stygian Owl 4. Ash-throated Antwren 5. Stripe-headed Antpitta 6. Ochre-fronted Antpitta 7. Grey-bellied Comet 8. Long-tailed Potoo 9. Jelski’s Chat-Tyrant 10. = Chestnut-backed Thornbird, Yellow-breasted Brush Finch You know it has been a good tour when neither Marvellous Spatuletail nor Long-whiskered Owlet make the top 10 of birds seen! Day 1: 4 September: Pacific coast and Chaparri Upon meeting, we headed straight towards the coast and birded the fields near Monsefue, quickly finding Coastal Miner. Our main quarry proved trickier and we had to scan a lot of fields before eventually finding a distant flock of Tawny-throated Dotterel; we walked closer, getting nice looks at a flock of 24 of the near-endemic pallidus subspecies of this cracking shorebird. -

Bolivia's Avian Riches 2016 BIRDS
Field Guides Tour Report Bolivia's Avian Riches 2016 Sep 3, 2016 to Sep 19, 2016 Dan Lane For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Seeing several Cream-backed Woodpeckers this well was definitely one of the highlights of the tour. Photo by participant Brian Stech. Bolivia, as per usual, didn’t disappoint with some amazing scenery, great birds, and the occasional logistical challenge. Roadwork caused a few delays but, on occasion, actually gave us an unexpected opportunity to see a good bird! That Hellmayr’s Pipit in Siberia and the side road to search (and successfully find!) Red-fronted Macaw and a few other targets along the Mizque were both happy results, for example. But the tour gave us some other great memories: high on the list were the astounding views of several Cream-backed Woodpeckers—a rather reasonable stand-in for the Ivory-billed! We also reveled in our luck with the difficult Scimitar-winged Piha, which showed in spades for us! The boldly-patterned White-eared Solitaire and the colorful Band-tailed Fruiteater were other birds that ranked high on our lists of species seen. Others we appreciated were the bold Brown Tinamou that crossed the track in front of us, the moxie of that male Andean Hillstar that inspected us up close, the majesty of the Andean Condors we saw on several days, the startling plumage of the Red-tailed Comet, and the equally captivating Black-hooded Sunbeam, the more muted beauty of the Cochabamba Mountain-Finch, and the escaped jailbird plumage of the Barred Fruiteater. -
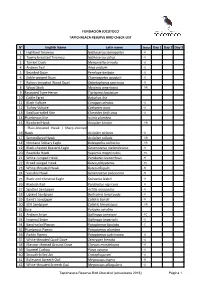
N° English Name Latin Name Status Day 1 Day 2
FUNDACIÓN JOCOTOCO TAPICHALACA RESERVE BIRD CHECK-LIST N° English Name Latin name Status Day 1 Day 2 Day 3 1 Highland Tinamou Nothocercus bonapartei - R 2 Tawny-breasted Tinamou Nothocercus julius - U 3 Torrent Duck Merganetta armata - U 4 Andean Teal Anas andium - U 5 Bearded Guan Penelope barbata - U 6 Sickle-winged Guan Chamaepetes goudotii - U 7 Rufous-breasted Wood Quail Odontophorus speciosus - R 8 Wood Stork Mycteria americana - VR 9 Fasciated Tiger-Heron Tigrisoma fasciatum 10 Cattle Egret Bubulcus ibis 11 Black Vulture Coragyps atratus - U 12 Turkey Vulture Cathartes aura - U 13 Swallow-tailed Kite Elanoides forficatus - U 14 Plumbeous Kite Ictinia plumbea 15 Bicolored Hawk Accipiter bicolor - VR Plain-breasted Hawk / Sharp-shinned 16 Hawk Accipiter striatus - U 17 Semicollared Hawk Accipiter collaris - VR 18 Montane Solitary Eagle Buteogallus solitarius - VR 19 Black-chested Buzzard-Eagle Geranoaetus melanoleucus - R 20 Roadside Hawk Rupornis magnirostris - FC 21 White-rumped Hawk Parabuteo leucorrhous - R 22 Broad-winged Hawk Buteo platypterus - FC 23 White-throated Hawk Buteo albigula - R 24 Variable Hawk Geranoaetus polyosoma - R 25 Black-and-chestnut Eagle Spizaetus isidori - R 26 Blackish Rail Pardirallus nigricans - R 27 Spotted Sandpiper Actitis macularius - R 28 Upland Sandpiper Bartramia longicauda - R 29 Baird's Sandpiper Calidris bairdii - R 30 Stilt Sandpiper Calidris himantopus - VR 31 Sora Porzana carolina 32 Andean Snipe Gallinago jamesoni - FC 33 Imperial Snipe Gallinago imperialis - U 34 Band-tailed Pigeon Patagioenas -

Peru: Manu and Machu Picchu August 2010
Peru: Manu and Machu Picchu August 2010 PERU: Manu and Machu Picchu 13 – 30 August 2010 Tour Leader: Jose Illanes Itinerary: August 13: Arrival day/ Night Lima August 14: Fly Lima-Cusco, Bird at Huacarpay Lake/ Night Cusco August 15: Upper Manu Road/Night Cock of the Rock Lodge August 16-17: San Pedro Area/ Nights cock of the Rock Lodge August 18: San Pedro-Atalaya/Night Amazonia Lodge August 19-20: Amazonia Lodge/Nights Amazonia Lodge August 21: River Trip to Manu Wildlife Center/Night Manu Wildlife Center August 22-24: Manu Wildlife Center /Nights Manu Wildlife Center August 25: Manu Wildlife Center - Boat trip to Puerto Maldonado/ Night Puerto Maldonado August 26: Fly Puerto Maldonado-Cusco/Night Ollantaytambo www.tropicalbirding.com Tropical Birding 1-409-515-0514 1 Peru: Manu and Machu Picchu August 2010 August 27: Abra Malaga Pass/Night Ollantaytambo August 28: Ollantaytambo-Machu Picchu/Night Aguas Calientes August 29: Aguas Calientes and return Cusco/Night Cusco August 30: Fly Cusco-Lima, Pucusana & Pantanos de Villa. Late evening departure. August 14 Lima to Cusco to Huacarpay Lake After an early breakfast in Peru’s capital Lima, we took a flight to the Andean city of Cusco. Soon after arriving in Cusco and meeting with our driver we headed out to Huacarpay Lake . Unfortunately our arrival time meant we got there when it was really hot, and activity subsequently low. Although we stuck to it, and slowly but surely, we managed to pick up some good birds. On the lake itself we picked out Puna Teal, Speckled and Cinnamon Teals, Andean (Slate-colored) Coot, White-tufted Grebe, Andean Gull, Yellow-billed Pintail, and even Plumbeous Rails, some of which were seen bizarrely swimming on the lake itself, something I had never seen before. -

Cotinga 1–10
COTINGA INDEX Numbers 1–10 (1994–1998) Compiled by Tom Stuart NEOTROPICAL BIRD CLUB Contents – Sumário – Contenido Authors and papers Autores e papéis Autores y artículos 4 Bird species Espécies de aves Especies de aves 11 Countries and territories Países e territórios Países y teritorios 20 Protected areas Áreas de proteção Áreas protegidas 22 NBC conservation awards NBC prêmios de conservação NBC premios de conservación 24 Plates Pranchas Láminas 25 Reviews Revisões Revisiones de literatura 30 Interpretation and tips – Interpretación y códigos All page numbers have a two-letter code as a guide to the nature of the reference. Todos los números de página llevan un código de dos letras como referencia a la información presentada en cada artículo. PP Paper (Artículo) PS Photospot SN Short note (Nota corta) CA NBC Conservation Award TR Taxonomic Round-up NB Neotropical Notebook NE Neotropical News Bird species are listed in the index if they appear in the title or the summary of a paper or other article, except in the case of the Neotropical Notebook, for which every species entry is included. Countries and territories are listed if they named in the title of a paper, short note, or other section of the journal. Protected areas are included where they are part of the title of a paper, Conservation Award or Neotropical News entry. The plates index includes photographs and painted plates, but not figures or line drawings. Se mencionan las especies de aves en el índice cuando aparecen en el título o el resumen de un artículo u otra nota, excepto en el caso del Neotropical Notebook, donde se incluye cada especie mencionada. -

University of Florida Thesis Or Dissertation
DISTRIBUTIONAL ECOLOGY AND DIVERSITY PATTERNS OF TROPICAL MONTANE BIRDS By JILL EMILY JANKOWSKI A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2010 1 © 2010 Jill Emily Jankowski 2 To my parents, Mike and Laverne, and my loving and adorable partner in crime, Aaron 3 ACKNOWLEDGMENTS This dissertation would not have been possible without the unwavering dedication and guidance from my co-advisors and committee, the long months of work contributed by my assistants, the logistical and financial support of many people and organizations, both in the United States and abroad, and the patience and encouragement from loved ones throughout this six-year endeavor. My advisors, Doug Levey and Scott Robinson, have been terrific role models in academics and teaching, and their dedication to family serves as an example of how to keep the most important things in perspective. The members of my committee, including R. Holt, S. Phelps, and K. Sieving, were extremely supportive and gave constructive feedback on all aspects of the dissertation. Many people worked in the field with me and assisted in data collection, often for months on end, including T. Boza, R. Butrón, L. M. Cabrera, R. Cruz, K. Elliot, V. Huamán, M. Jurado, M. Libsch, G. Londoño, J. Olano, Z. Peterson, N. Quisiyupanqui, R. Quispes, M. Salazar, S. J. Socolar, A. Spalding, J. Ungvari-Martin, and C. Zurita. I thank the Campbell, Cruz, Guindon, Leitón, Lowther, Morrison, Rockwell and Stuckey families of Monteverde for allowing me to work on their properties, and the Monteverde and Santa Elena cloudforest preserves, the Monteverde Conservation League, Bosque Eterno, and Monteverde Biological Station for granting permission to work in these protected areas.