On Microrna and the Need for Exploratory Experimentation in Post
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

USA Education Ph.D., Biology, Massachusetts Institute of Tech
Victor R. Ambros, Ph.D. Silverman Professor of Natural Sciences Program in Molecular Medicine University of Massachusetts Medical School373 Plantation Street, Suite 306 Worcester, MA 01605 (508) 856-6380 [email protected] Personal Born: Hanover, NH, USA on December 1, 1953 Citizenship: USA Education Ph.D., Biology, Massachusetts Institute of Technology, Cambridge, MA 1976-1979 Thesis Title: The protein covalently linked to the 5' end of poliovirus RNA Advisor: Dr. David Baltimore B.S., Biology, Massachusetts Institute of Technology, Cambridge, MA 1971-1975 Professional Appointments Silverman Professor of Natural Sciences 2009-present Co-Director, RNA Therapeutics Institute 2009-2016 Professor, Program in Molecular Medicine 2008-present University of Massachusetts Medical School, Worcester, MA Professor of Genetics, Dartmouth Medical School 2001-2007 Professor, Biological Sciences, Dartmouth Medical School 1996-2001 Associate Professor, Biological Sciences, Dartmouth Medical School 1992-1996 Associate Professor, Department of Cellular and Development Biology, 1988-1992 Harvard University, Cambridge, MA Assistant Professor, Department of Cellular and Development Biology, 1985-1988 Harvard University, Cambridge, MA Postdoctoral Research 1979-1985 Supervisor: Dr. H. Robert Horvitz Massachusetts Institute of Technology, Cambridge, MA Graduate Research 1976-1979 Supervisor: Dr. David Baltimore Massachusetts Institute of Technology, Cambridge, MA Research Assistant 1975-1976 Supervisor: Dr. David Baltimore Center for Cancer Research, -

Francois Jacob Memorial
RETROSPECTIVE RETROSPECTIVE Francois Jacob memorial Arthur B. Pardee1 Department of Adult Oncology, Dana-Farber Institute, Boston, MA 02115 Dr. Francois Jacob is one of a handful of the DNA would integrate into the bacterial chro- 20th century’smostdistinguishedlifescien- mosome and remain dormant or, at other tists. His research with Dr. Jacques Monod, times, would kill the cell. like that of Watson and Crick, provided the Jacob’s next major contribution, in collab- foundations for understanding mechanisms oration with Dr. Jacques Monod, was to in- of genetic regulation of life processes such vestigate how a gene is regulated. Remark- as cell differentiation and defects in diseases. ably, native E. coli synthesize β-galactosidase Jacob joined the College de France in 1964 only when lactose is available. Some mutated and shared the Nobel Prize in Physiology bacteria can make the enzyme in the absence or Medicine 1965 with Jacques Monod of inducer. Monod’s initial idea was that and Andre Lwoff. He was elected to the these constitutive bacteria activate the gene National Academy of Sciences (NAS) USA by synthesizing an intracellular lactose-like in 1969. inducer molecule. Jacob was born in 1920 in a French Jewish To investigate this model, interrupted family; his grandfather was a four-star gen- mating was applied to bring the β-galactosi- eral. He began to study medicine before dase gene of a donor bacterium into a consti- World War II, in which he served as a mil- tutive receptor. According to the induction itary officer in the Free French Army and was model, the mated cell should produce en- badly wounded in an air raid. -

Nature Medicine Essay
COMMENTARY LASKER BASIC MEDICAL RESEARCH AWARD Of maize and men, or peas and people: case histories to justify plants and other model systems David Baulcombe One of the byproducts of molecular biology cork is altogether filled with air, and that air is has been support for the ‘model system’ con- perfectly enclosed in little boxes or cells distinct cept. All living organisms are based on the same from one another.”)2 (Fig. 1). Two hundred fifty genetic code, they have similar subcellular years later, Beijerinck discovered a contagium structures and they use homologous metabolic vivum fluidum in extracts of diseased tobacco pathways. So, mechanisms can be investigated plants that he later referred to as a virus3. using organisms other than those in which In contemporary science, a green alga— the knowledge will be exploited for practical Chlamydomonas reinhardtii—is a useful model benefit. Model systems are particularly use- in the analysis of kidney disease4. However, ful in the early discovery phase of a scientific in this article, I refer to the contribution of endeavor, and recent progress in biomedical plant biology to a family of mechanisms that I science has fully vindicated their use. Jacques refer to as RNA silencing. This topic has been Monod, for example, famously justified his reviewed comprehensively elsewhere5,6, so here work on a bacterial model system by stating I focus on personal experience and my view of that “what is true for Escherichia coli is also future potential from this work. true for elephants.” My fellow laureates, Victor Ambros and Gary Ruvkun, can defend the use The early history of RNA silencing in of the worm Caenorhabditis elegans as a good plants model system and so I will focus on plants. -

Quiet Debut'' of the Double Helix: a Bibliometric and Methodological
Journal of the History of Biology Ó Springer 2009 DOI 10.1007/s10739-009-9183-2 Revisiting the ‘‘Quiet Debut’’ of the Double Helix: A Bibliometric and Methodological note on the ‘‘Impact’’ of Scientific Publications YVES GINGRAS De´partement d’histoire Universite´ du Que´bec a` Montre´al C.P. 8888, Suc. Centre-Ville Montreal, QC H3C-3P8 Canada E-mail: [email protected] Abstract. The object of this paper is two-fold: first, to show that contrary to what seem to have become a widely accepted view among historians of biology, the famous 1953 first Nature paper of Watson and Crick on the structure of DNA was widely cited – as compared to the average paper of the time – on a continuous basis from the very year of its publication and over the period 1953–1970 and that the citations came from a wide array of scientific journals. A systematic analysis of the bibliometric data thus shows that Watson’s and Crick’s paper did in fact have immediate and long term impact if we define ‘‘impact’’ in terms of comparative citations with other papers of the time. In this precise sense it did not fall into ‘‘relative oblivion’’ in the scientific community. The second aim of this paper is to show, using the case of the reception of the Watson–Crick and Jacob–Monod papers as concrete examples, how large scale bibliometric data can be used in a sophisticated manner to provide information about the dynamic of the scientific field as a whole instead of limiting the analysis to a few major actors and generalizing the result to the whole community without further ado. -

Ethical Principles in Ethical Principles in Scientific Research Scientific Research and Publications
Hacettepe University Institute of Oncology Library ETHICAL PRINCIPLES IN SCIENTIFIC LectureRESEARCH author AND PUBLICATIONSOnlineby © Emin Kansu,M.D.,FACP ESCMID ekansu@ada. net. tr Library Lecture author Onlineby © ESCMID HACETTEPE UNIVERSITY MEDICAL CENTER Library Lecture author Onlineby © ESCMID INSTITUTE OF ONCOLOGY - HACETTEPE UNIVERSITY Ankara PRESENTATION • UNIVERSITY and RESEARCHLibrary • ETHICS – DEFINITION • RESEARCH ETHICS • PUBLICATIONLecture ETHICS • SCIENTIFIC MISCONDUCTauthor • SCIENTIFIC FRAUD AND TYPES Onlineby • HOW TO PREVENT© UNETHICAL ISSUES • WHAT TO DO FOR SCIENTIFIC ESCMIDMISCONDUCTS Library Lecture author Onlineby © ESCMID IMPACT OF TURKISH SCIENTISTS 85 % University – Based 1.78% 0.2 % 19. 300 18th ‘ACADEMIA’ UNIVERSITY Library AN INSTITUTION PRODUCING and DISSEMINATING SCIENCE Lecture BASIC FUNCTIONS author Online-- EDUCATIONby © -- RESEARCH ESCMID - SERVICESERVICE UNIVERSITY Library • HAS TO BE OBJECTIVE • HAS TO BE HONESTLecture AND ETHICAL • HAS TO PERFORM THEauthor “STATE-OF-THE ART” • HAS TO PLAYOnline THEby “ROLE MODEL” , TO BE GENUINE AND ©DISCOVER THE “NEW” • HAS TO COMMUNICATE FREELY AND HONESTLY WITH ALL THE PARTIES INVOLVED IN ESCMIDPUBLIC WHY WE DO RESEARCH ? Library PRIMARY AIM - ORIGINAL CONTRIBUTIONS TO SCIENCE Lecture SECONDARY AIM author - TOOnline PRODUCEby A PAPER - ACADEMIC© PROMOTION - TO OBTAIN AN OUTSIDE SUPPORT ESCMID SCIENTIFIC RESEARCH Library A Practice aimed to contribute to knowledge or theoryLecture , performed in disciplined methodologyauthor and Onlineby systematic approach© -

PIE-1 Sumoylation Promotes Germline Fates and Pirna-Dependent Silencing in C
RESEARCH ARTICLE PIE-1 SUMOylation promotes germline fates and piRNA-dependent silencing in C. elegans Heesun Kim1†, Yue-He Ding1†, Shan Lu2, Mei-Qing Zuo2, Wendy Tan1, Darryl Conte Jr1, Meng-Qiu Dong2, Craig C Mello1,3* 1RNA Therapeutics Institute, University of Massachusetts Medical School, Worcester, United States; 2National Institute of Biological Sciences, Beijing, China; 3Howard Hughes Medical Institute, Chevy Chase, United States Abstract Germlines shape and balance heredity, integrating and regulating information from both parental and foreign sources. Insights into how germlines handle information have come from the study of factors that specify or maintain the germline fate. In early Caenorhabditis elegans embryos, the CCCH zinc finger protein PIE-1 localizes to the germline where it prevents somatic differentiation programs. Here, we show that PIE-1 also functions in the meiotic ovary where it becomes SUMOylated and engages the small ubiquitin-like modifier (SUMO)-conjugating machinery. Using whole-SUMO-proteome mass spectrometry, we identify HDAC SUMOylation as a target of PIE-1. Our analyses of genetic interactions between pie-1 and SUMO pathway mutants suggest that PIE-1 engages the SUMO machinery both to preserve the germline fate in the embryo and to promote Argonaute-mediated surveillance in the adult germline. *For correspondence: Introduction [email protected] During every life cycle, the eukaryotic germline orchestrates a remarkable set of informational tasks †These authors contributed that shape heredity and create variation necessary for the evolution of new species. One approach equally to this work for understanding the mechanisms that promote germline specification and function has been the identification of genes whose protein products localize exclusively to the germline and for which Competing interests: The loss-of-function mutations result in absent or non-functional germ cells and gametes (Seydoux and authors declare that no Braun, 2006). -

Fire Departments of Pathology and Genetics, Stanford University School of Medicine, 300 Pasteur Drive, Room L235, Stanford, CA 94305-5324, USA
GENE SILENCING BY DOUBLE STRANDED RNA Nobel Lecture, December 8, 2006 by Andrew Z. Fire Departments of Pathology and Genetics, Stanford University School of Medicine, 300 Pasteur Drive, Room L235, Stanford, CA 94305-5324, USA. I would like to thank the Nobel Assembly of the Karolinska Institutet for the opportunity to describe some recent work on RNA-triggered gene silencing. First a few disclaimers, however. Telling the full story of gene silencing would be a mammoth enterprise that would take me many years to write and would take you well into the night to read. So we’ll need to abbreviate the story more than a little. Second (and as you will see) we are only in the dawn of our knowledge; so consider the following to be primer... the best we could do as of December 8th, 2006. And third, please understand that the story that I am telling represents the work of several generations of biologists, chemists, and many shades in between. I’m pleased and proud that work from my labo- ratory has contributed to the field, and that this has led to my being chosen as one of the messengers to relay the story in this forum. At the same time, I hope that there will be no confusion of equating our modest contributions with those of the much grander RNAi enterprise. DOUBLE STRANDED RNA AS A BIOLOGICAL ALARM SIGNAL These disclaimers in hand, the story can now start with a biography of the first main character. Double stranded RNA is probably as old (or almost as old) as life on earth. -
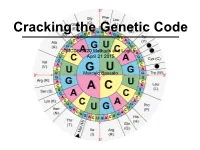
MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo
Cracking the Genetic Code MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) Frederick Griffith The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) Oswald Avery Alfred Hershey Martha Chase The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) Linus Carl Pauling The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) James Watson and Francis Crick The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) How is DNA (4 nucleotides) the genetic material while proteins (20 amino acids) are the building blocks? ? DNA Protein ? The Coding Craze ? DNA Protein What was already known? • DNA resides inside the nucleus - DNA is not the carrier • Protein synthesis occur in the cytoplasm through ribosomes {• Only RNA is associated with ribosomes (no DNA) - rRNA is not the carrier { • Ribosomal RNA (rRNA) was a homogeneous population The “messenger RNA” hypothesis François Jacob Jacques Monod The Coding Craze ? DNA RNA Protein RNA Tie Club Table from Wikipedia The Coding Craze Who won the race Marshall Nirenberg J. -

The Eighth Day of Creation”: Looking Back Across 40 Years to the Birth of Molecular Biology and the Roots of Modern Cell Biology
“The Eighth Day of Creation”: looking back across 40 years to the birth of molecular biology and the roots of modern cell biology Mark Peifer1 1 Department of Biology and Curriculum in Genetics and Molecular Biology, University of North Carolina at Chapel Hill, CB#3280, Chapel Hill, NC 27599-3280, USA * To whom correspondence should be addressed Email: [email protected] Phone: (919) 962-2272 1 Forty years ago, Horace Judson’s “The Eight Day of Creation” was published, a book vividly recounting the foundations of modern biology, the molecular biology revolution. This book inspired many in my generation. The anniversary provides a chance for a new generation to take a look back, to see how science has changed and hasn’t changed. Many central players in the book, including Sydney Brenner, Seymour Benzer and Francois Jacob, would go on to be among the founders of modern cell, developmental, and neurobiology. These players come alive via their own words, as complex individuals, both heroes and anti-heroes. The technologies and experimental approaches they pioneered, ranging from cell fractionation to immunoprecipitation to structural biology, and the multidisciplinary approaches they took continue to power and inspire our work today. In the process, Judson brings out of the shadows the central roles played by women in many of the era’s discoveries. He provides us with a vision of how science and scientists have changed, of how many things about our endeavor never change, and how some new ideas are perhaps not as new as we’d like to think. 2 In 1979 Horace Judson completed a ten-year project about cell and molecular biology’s foundations, unveiling “The Eighth Day of Creation”, a book I view as one of the most masterful evocations of a scientific revolution (Judson, 1979). -

In 1953 in England James Watson and Francis Crick Discovered the Structure of DNA in the Now-Famous Scientific Narrative Known As the “Race Towards the Double Helix”
THE NARRATIVES OF SCIENCE: LITERARY THEORY AND DISCOVERY IN MOLECULAR BIOLOGY PRIYA VENKATESAN In 1953 in England James Watson and Francis Crick discovered the structure of DNA in the now-famous scientific narrative known as the “race towards the double helix”. Meanwhile in France, Roland Barthes published his first book, Writing Degree Zero, on literary theory, which became the intellectual precursor for the new human sciences that were developing based on Saussurean linguistics. The discovery by Watson and Crick of the double helix marked a definitive turning point in the development of the life sciences, paving the way for the articulation of the genetic code and the emergence of molecular biology. The publication by Barthes was no less significant, since it served as an exemplar for elucidating how literary narratives are structured and for formulating how textual material is constructed. As Françoise Dosse notes, Writing Degree Zero “received unanimous acclaim and quickly became a symptom of new literary demands, a break with tradition”.1 Both the work of Roland Barthes and Watson and Crick served as paradigms in their respective fields. Semiotics, the field of textual analysis as developed by Barthes in Writing Degree Zero, offered a new direction in the structuring of narrative whereby each distinct unit in a story formed a “code” or “isotopy” that categorizes the formal elements of the story. The historical concurrence of the discovery of the double helix and the publication of Writing Degree Zero may be mere coincidence, but this essay is an exploration of the intellectual influence that both events may have had on each other, since both the discovery of the double helix and Barthes’ publication gave expression to the new forms of knowledge 1 Françoise Dosse, History of Structuralism: The Rising Sign, 1945-1966, trans. -

Cover June 2011
z NOBEL LAUREATES IN Qui DNA RESEARCH n u SANGRAM KESHARI LENKA & CHINMOYEE MAHARANA F 1. Who got the Nobel Prize in Physiology or Medicine 1933) for discovering the famous concept that says chromosomes carry genes? a. Gregor Johann Mendel b. Thomas Hunt Morgan c. Aristotle d. Charles Darwin 5. Name the Nobel laureate (1959) for his discovery of the mechanisms in the biological 2. The concept of Mutations synthesis of ribonucleic acid and are changes in genetic deoxyribonucleic acid? information” awarded him a. Arthur Kornberg b. Har Gobind Khorana the Nobel Prize in 1946: c. Roger D. Kornberg d. James D. Watson a. Hermann Muller b. M.F. Perutz c. James D. Watson 6. Discovery of the DNA double helix fetched them d. Har Gobind Khorana the Nobel Prize in Physiology or Medicine (1962). a. Francis Crick, James Watson, Rosalind Elsie Franklin b. Francis Crick, James Watson and Maurice Willkins c. James Watson, Maurice Willkins, Rosalind Elsie Franklin 3. Identify the discoverer and d. Maurice Willkins, Rosalind Elsie Franklin and Francis Crick Nobel laureate of 1958 who found DNA in bacteria and viruses. a. Louis Pasteur b. Alexander Fleming c. Joshua Lederberg d. Roger D. Kornberg 4. A direct link between genes and enzymatic reactions, known as the famous “one gene, one enzyme” hypothesis, was put forth by these 7. They developed the theory of genetic regulatory scientists who shared the Nobel Prize in mechanisms, showing how, on a molecular level, Physiology or Medicine, 1958. certain genes are activated and suppressed. Name a. George Wells Beadle and Edward Lawrie Tatum these famous Nobel laureates of 1965. -

Balcomk41251.Pdf (558.9Kb)
Copyright by Karen Suzanne Balcom 2005 The Dissertation Committee for Karen Suzanne Balcom Certifies that this is the approved version of the following dissertation: Discovery and Information Use Patterns of Nobel Laureates in Physiology or Medicine Committee: E. Glynn Harmon, Supervisor Julie Hallmark Billie Grace Herring James D. Legler Brooke E. Sheldon Discovery and Information Use Patterns of Nobel Laureates in Physiology or Medicine by Karen Suzanne Balcom, B.A., M.L.S. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin August, 2005 Dedication I dedicate this dissertation to my first teachers: my father, George Sheldon Balcom, who passed away before this task was begun, and to my mother, Marian Dyer Balcom, who passed away before it was completed. I also dedicate it to my dissertation committee members: Drs. Billie Grace Herring, Brooke Sheldon, Julie Hallmark and to my supervisor, Dr. Glynn Harmon. They were all teachers, mentors, and friends who lifted me up when I was down. Acknowledgements I would first like to thank my committee: Julie Hallmark, Billie Grace Herring, Jim Legler, M.D., Brooke E. Sheldon, and Glynn Harmon for their encouragement, patience and support during the nine years that this investigation was a work in progress. I could not have had a better committee. They are my enduring friends and I hope I prove worthy of the faith they have always showed in me. I am grateful to Dr.