Supplementary Materials For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SUSANNE BRUNKHORST, D V M (B)(6), (B)(7)(C)
SBRUNKHORST United States Department of Agriculture Animal and Plant Health Inspection Service 2016082567931581 Insp_id Inspection Report University Of Tennesee - Chattanooga Customer ID: 29 975 East Third St. Certificate: 63-R-0001 Box 339 Site: 001 College Of Medicine COLLEGE OF MEDICINE Chattanooga, TN 37403 Type: ROUTINE INSPECTION Date: 20-SEP-2016 No non-compliant items identified during this inspection. This inspection and exit interview were conducted with undersigned facility representative. SUSANNE BRUNKHORST, D V M Prepared By: Date: SUSANNE BRUNKHORST USDA, APHIS, Animal Care 20-SEP-2016 Title: VETERINARY MEDICAL OFFICER 1076 Received By: (b)(6), (b)(7)(c) Date: Title: FACILITY REPRESENTATIVE 20-SEP-2016 Page 1 of 1 United States Department of Agriculture Customer: 29 Animal and Plant Health Inspection Service Inspection Date: 20-SEP-16 Animal Inspected at Last Inspection Cust No Cert No Site Site Name Inspection 29 63-R-0001 001 UNIVERSITY OF TENNESEE - 20-SEP-16 CHATTANOOGA Count Species 000000 None 000000 Total United States Department of Agriculture Customer: 29 Animal and Plant Health Inspection Service Inspection Date: 24-AUG-15 Animal Inspected at Last Inspection Cust No Cert No Site Site Name Inspection 29 63-R-0001 001 UNIVERSITY OF TENNESEE - 24-AUG-15 CHATTANOOGA Count Species 000000 None 000000 Total United States Department of Agriculture Customer: 851 Animal and Plant Health Inspection Service Inspection Date: 04-FEB-16 Animal Inspected at Last Inspection Cust No Cert No Site Site Name Inspection 851 63-R-0002 -

The Taxonomy of Primates in the Laboratory Context
P0800261_01 7/14/05 8:00 AM Page 3 C HAPTER 1 The Taxonomy of Primates T HE T in the Laboratory Context AXONOMY OF P Colin Groves RIMATES School of Archaeology and Anthropology, Australian National University, Canberra, ACT 0200, Australia 3 What are species? D Taxonomy: EFINITION OF THE The biological Organizing nature species concept Taxonomy means classifying organisms. It is nowadays commonly used as a synonym for systematics, though Disagreement as to what precisely constitutes a species P strictly speaking systematics is a much broader sphere is to be expected, given that the concept serves so many RIMATE of interest – interrelationships, and biodiversity. At the functions (Vane-Wright, 1992). We may be interested basis of taxonomy lies that much-debated concept, the in classification as such, or in the evolutionary implica- species. tions of species; in the theory of species, or in simply M ODEL Because there is so much misunderstanding about how to recognize them; or in their reproductive, phys- what a species is, it is necessary to give some space to iological, or husbandry status. discussion of the concept. The importance of what we Most non-specialists probably have some vague mean by the word “species” goes way beyond taxonomy idea that species are defined by not interbreeding with as such: it affects such diverse fields as genetics, biogeog- each other; usually, that hybrids between different species raphy, population biology, ecology, ethology, and bio- are sterile, or that they are incapable of hybridizing at diversity; in an era in which threats to the natural all. Such an impression ultimately derives from the def- world and its biodiversity are accelerating, it affects inition by Mayr (1940), whereby species are “groups of conservation strategies (Rojas, 1992). -

NO2N Import Into Containment Any New Organism That Is Not Genetically Modified
NO2N Import into containment any new organism that is not genetically modified Application title: Importation of specified “new” mammal species into containment at Wellington Zoo, and other zoos, to aid conservation though sustainable display, captive breeding and / or the conservation of genetic material Applicant organisation: Wellington Zoo Trust, 200 Daniell Street, Newtown, Wellington Please provide a brief summary of the purpose of the application (255 characters or less, including spaces) To import into containment 28 mammal species for captive breeding, display, educational presentations and to contribute to conservation by exposing visitors to conservation issues and the conservation of genetic material through breeding PLEASE CONTACT ERMA NEW ZEALAND BEFORE SUBMITTING YOUR APPLICATION Please clearly identify any confidential information and attach as a separate appendix. Please check and complete the following before submitting your application: All sections completed Yes Appendices enclosed NA Confidential information identified and enclosed separately NA Copies of references attached Yes Application signed and dated Yes Electronic copy of application e-mailed to Yes ERMA New Zealand Signed: Date: 20 Customhouse Quay Cnr Waring Taylor and Customhouse Quay PO Box 131, Wellington Phone: 04 916 2426 Fax: 04 914 0433 Email: [email protected] Website: www.ermanz.govt.nz NO2N: Application to import into containment any new organism that is not genetically modified Section One – Applicant details Name and details of the organisation -

Evolutionary Stasis of the Pseudoautosomal Boundary In
Evolutionary stasis of the pseudoautosomal boundary in strepsirrhine primates Rylan Shearn, Alison E Wright, Sylvain Mousset, Corinne Régis, Simon Penel, Jean-François Lemaître, Guillaume Douay, Brigitte Crouau-Roy, Emilie Lecompte, Gabriel Ab Marais To cite this version: Rylan Shearn, Alison E Wright, Sylvain Mousset, Corinne Régis, Simon Penel, et al.. Evolutionary stasis of the pseudoautosomal boundary in strepsirrhine primates. eLife, eLife Sciences Publication, 2020, 9, 10.7554/eLife.63650. hal-03064964 HAL Id: hal-03064964 https://hal.archives-ouvertes.fr/hal-03064964 Submitted on 14 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. SHORT REPORT Evolutionary stasis of the pseudoautosomal boundary in strepsirrhine primates Rylan Shearn1, Alison E Wright2, Sylvain Mousset1,3, Corinne Re´ gis1, Simon Penel1, Jean-Franc¸ois Lemaitre1, Guillaume Douay4, Brigitte Crouau-Roy5, Emilie Lecompte5, Gabriel AB Marais1,6* 1Laboratoire Biome´trie et Biologie Evolutive, CNRS / Univ. Lyon 1, Villeurbanne, France; 2Department of Animal and Plant Sciences, University of Sheffield, Sheffield, United Kingdom; 3Faculty of Mathematics, University of Vienna, Vienna, Austria; 4Zoo de Lyon, Lyon, France; 5Laboratoire Evolution et Diversite´ Biologique, CNRS / Univ. Toulouse, Toulouse, France; 6LEAF-Linking Landscape, Environment, Agriculture and Food Dept, Instituto Superior de Agronomia, Universidade de Lisboa, Lisbon, Portugal Abstract Sex chromosomes are typically comprised of a non-recombining region and a recombining pseudoautosomal region. -

Animals Traded for Traditional Medicine at the Faraday Market in South Africa: Species Diversity and Conservation Implications M
Journal of Zoology Journal of Zoology. Print ISSN 0952-8369 Animals traded for traditional medicine at the Faraday market in South Africa: species diversity and conservation implications M. J. Whiting1,2, V. L. Williams1 & T. J. HibbittsÃ,1 1 School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa 2 Department of Biological Sciences, Macquarie University, Sydney, Australia Keywords Abstract biodiversity; threatened species; ethnozoology; mammal; bird; reptile. In South Africa, animals and plants are commonly used as traditional medicine for both the healing of ailments and for symbolic purposes such as improving Correspondence relationships and attaining good fortune. The aim of this study was twofold: to Department of Biological Sciences, quantify the species richness and diversity of traded animal species and to assess Macquarie University, Sydney, NSW 2109, the trade in species of conservation concern. We surveyed the Faraday traditional Australia. medicine market in Johannesburg and conducted 45 interviews of 32 traders Email: [email protected] during 23 visits. We identified 147 vertebrate species representing about 9% of the total number of vertebrate species in South Africa and about 63% of the total ÃCurrent address: Department of Wildlife number of documented species (excluding domestic animals) traded in all South and Fisheries Sciences, Texas A & M African traditional medicine markets. The vertebrates included 60 mammal University, College Station, TX 77843-2258, species, 33 reptile species, 53 bird species and one amphibian species. Overall, USA. species diversity in the Faraday market was moderately high and highest for mammals and birds, respectively. Evenness values indicated that relatively few Editor: Andrew Kitchener species were dominant. -

Chapter 15 the Mammals of Angola
Chapter 15 The Mammals of Angola Pedro Beja, Pedro Vaz Pinto, Luís Veríssimo, Elena Bersacola, Ezequiel Fabiano, Jorge M. Palmeirim, Ara Monadjem, Pedro Monterroso, Magdalena S. Svensson, and Peter John Taylor Abstract Scientific investigations on the mammals of Angola started over 150 years ago, but information remains scarce and scattered, with only one recent published account. Here we provide a synthesis of the mammals of Angola based on a thorough survey of primary and grey literature, as well as recent unpublished records. We present a short history of mammal research, and provide brief information on each species known to occur in the country. Particular attention is given to endemic and near endemic species. We also provide a zoogeographic outline and information on the conservation of Angolan mammals. We found confirmed records for 291 native species, most of which from the orders Rodentia (85), Chiroptera (73), Carnivora (39), and Cetartiodactyla (33). There is a large number of endemic and near endemic species, most of which are rodents or bats. The large diversity of species is favoured by the wide P. Beja (*) CIBIO-InBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Vairão, Portugal CEABN-InBio, Centro de Ecologia Aplicada “Professor Baeta Neves”, Instituto Superior de Agronomia, Universidade de Lisboa, Lisboa, Portugal e-mail: [email protected] P. Vaz Pinto Fundação Kissama, Luanda, Angola CIBIO-InBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus de Vairão, Vairão, Portugal e-mail: [email protected] L. Veríssimo Fundação Kissama, Luanda, Angola e-mail: [email protected] E. -

Greater Galagos Near Mt. Kasigau, Kenya: Population Density Estimates Andrea Falcetto Western Kentucky University, [email protected]
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by TopSCHOLAR Western Kentucky University TopSCHOLAR® Masters Theses & Specialist Projects Graduate School 8-1-2012 Greater Galagos near Mt. Kasigau, Kenya: Population Density Estimates Andrea Falcetto Western Kentucky University, [email protected] Follow this and additional works at: http://digitalcommons.wku.edu/theses Part of the Biology Commons, and the Population Biology Commons Recommended Citation Falcetto, Andrea, "Greater Galagos near Mt. Kasigau, Kenya: Population Density Estimates" (2012). Masters Theses & Specialist Projects. Paper 1187. http://digitalcommons.wku.edu/theses/1187 This Thesis is brought to you for free and open access by TopSCHOLAR®. It has been accepted for inclusion in Masters Theses & Specialist Projects by an authorized administrator of TopSCHOLAR®. For more information, please contact [email protected]. GREATER GALAGOS NEAR MT. KASIGAU, KENYA: POPULATION DENSITY ESTIMATES A Thesis Presented to The Faculty of the Department of Biology Western Kentucky University Bowling Green, Kentucky In Partial Fulfillment of the Requirements for the Degree Master of Science By Andrea Falcetto August 2012 ACKNOWLEDGMENTS There are many individuals who assisted me with this project. I am thankful for everyone in the community of Kasigau, Kenya who helped with the project or simply made me feel welcome in the community. Specifically, my research assistants who provided their time to help me with distance sampling and trapping; these individuals were: Ducan Pesa, Kevin Kinyori and Ezara Kale. Ruphence Malemba provided friendship and support throughout the project including helping me find research assistants. Lastly, Simon Kagwa Kasaine helped tremendously with my research and made life in Kasigau a little easier. -

Animal Inspected at Last Inspection
United States Department of Agriculture Customer: 3432 Animal and Plant Health Inspection Service Inspection Date: 10-AUG-16 Animal Inspected at Last Inspection Cust No Cert No Site Site Name Inspection 3432 86-C-0001 001 ARIZONA CENTER FOR NATURE 10-AUG-16 CONSERVATION Count Species 000003 Cheetah 000005 Cattle/cow/ox/watusi 000003 Mandrill *Male 000006 Hamadryas baboon 000004 Grevys zebra 000008 Thomsons gazelle 000002 Cape Porcupine 000002 Lion 000002 African hunting dog 000002 Tiger 000008 Common eland 000002 Spotted hyena 000001 White rhinoceros 000007 Spekes gazelle 000005 Giraffe 000004 Kirks dik-dik 000002 Fennec fox 000003 Ring-tailed lemur 000069 Total ARHYNER United States Department of Agriculture Animal and Plant Health Inspection Service 2016082567967934 Insp_id Inspection Report Arizona Center For Nature Conservation Customer ID: 3432 455 N. Galvin Parkway Certificate: 86-C-0001 Phoenix, AZ 85008 Site: 001 ARIZONA CENTER FOR NATURE CONSERVATION Type: ROUTINE INSPECTION Date: 19-OCT-2016 No non-compliant items identified during this inspection. This inspection and exit interview were conducted with the primate manager. Additional Inspectors Gwendalyn Maginnis, Veterinary Medical Officer AARON RHYNER, D V M Prepared By: Date: AARON RHYNER USDA, APHIS, Animal Care 19-OCT-2016 Title: VETERINARY MEDICAL OFFICER 6077 Received By: (b)(6), (b)(7)(c) Date: Title: FACILITY REPRESENTATIVE 19-OCT-2016 Page 1 of 1 United States Department of Agriculture Customer: 3432 Animal and Plant Health Inspection Service Inspection Date: 19-OCT-16 -

Otolemur Crassicaudatus – Thick-Tailed Bushbaby
Otolemur crassicaudatus – Thick-tailed Bushbaby size (adult body weight 800–1500 g), relatively flat basicranium, low-crowned molars and reduced third upper molars (M3). Both species have a cornified region of skin proximal to the plantar pads of the hind foot that is rubbed noisily against the substrate during periods of agitation or insecurity. The distal margins of the nails are convex in young animals, but wear quickly to form a semi- lunar, concave margin in adults. The subspecies, O. c. crassicaudatus (Groves 2001), occurs in Mpumalanga, Limpopo and KwaZulu-Natal provinces in South Africa and Mozambique south of the Limpopo River. Across this region the pelage is variable, ranging from dark grey to chocolate brown, and various admixtures of L. Pozzi the two. Pelage variation is likely to be linked to varying needs for camouflage in different vegetation types, and possibly different seasons, as the animals are most Regional Red List status (2016) Least Concern vulnerable to predation when asleep during the day. National Red List status (2004) Least Concern Reasons for change No change Assessment Rationale Global Red List status (2008) Least Concern Listed as Least Concern, as the species is relatively widespread within the assessment region and in sub- TOPS listing (NEMBA) (2007) None Saharan Africa, and present in several protected areas in CITES listing (1977) Appendix II South Africa (including Kruger National Park). Northern and northeastern South Africa, however, comprise the Endemic No southernmost limit of its distribution, within which the species is confined to moist savannah and riverine forest, This very vocal species is the origin of the term which are naturally fragmented habitats, and Thick-tailed “bushbaby”; its repetitive cry sounds much like Bushbaby subpopulations are highly localised. -

Evolutionary History of Lorisiform Primates
Evolution: Reviewed Article Folia Primatol 1998;69(suppl 1):250–285 oooooooooooooooooooooooooooooooo Evolutionary History of Lorisiform Primates D. Tab Rasmussen, Kimberley A. Nekaris Department of Anthropology, Washington University, St. Louis, Mo., USA Key Words Lorisidae · Strepsirhini · Plesiopithecus · Mioeuoticus · Progalago · Galago · Vertebrate paleontology · Phylogeny · Primate adaptation Abstract We integrate information from the fossil record, morphology, behavior and mo- lecular studies to provide a current overview of lorisoid evolution. Several Eocene prosimians of the northern continents, including both omomyids and adapoids, have been suggested as possible lorisoid ancestors, but these cannot be substantiated as true strepsirhines. A small-bodied primate, Anchomomys, of the middle Eocene of Europe may be the best candidate among putative adapoids for status as a true strepsirhine. Recent finds of Eocene primates in Africa have revealed new prosimian taxa that are also viable contenders for strepsirhine status. Plesiopithecus teras is a Nycticebus- sized, nocturnal prosimian from the late Eocene, Fayum, Egypt, that shares cranial specializations with lorisoids, but it also retains primitive features (e.g. four premo- lars) and has unique specializations of the anterior teeth excluding it from direct lorisi- form ancestry. Another unnamed Fayum primate resembles modern cheirogaleids in dental structure and body size. Two genera from Oman, Omanodon and Shizarodon, also reveal a mix of similarities to both cheirogaleids and anchomomyin adapoids. Resolving the phylogenetic position of these Africa primates of the early Tertiary will surely require more and better fossils. By the early to middle Miocene, lorisoids were well established in East Africa, and the debate about whether these represent lorisines or galagines is reviewed. -
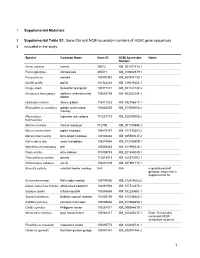
1 Supplemental Materials 1 Supplemental Table S1. Gene Ids and NCBI Accession Numbers of ACE2 Gene Sequences 2 Included In
1 Supplemental Materials 2 Supplemental Table S1. Gene IDs and NCBI accession numbers of ACE2 gene sequences 3 included in the study. Species Common Name Gene ID NCBI Accession Notes Number Homo sapiens human 59272 NM_001371415.1 Pan troglodytes chimpanzee 465511 XM_016942979.1 Pan paniscus bonobo 100970340 XM_008974180.1 Gorilla gorilla gorilla 101142534 XM_019019204.1 Pongo abelii Sumatran orangutan 100171441 NM_001131132.2 Nomascus leucogenys northern white-cheeked 100602708 XM_003261084.3 gibbon Hylobates moloch silvery gibbon 116811532 XM_032756617.1 Rhinopithecus roxellana golden snub-nosed 104664530 XM_010366065.2 monkey Piliocolobus Ugandan red colobus 111531712 XM_023199053.2 tephrosceles Macaca mulatta rhesus macaque 712790 NM_001135696.1 Macaca nemestrina pigtail macaque 105478157 XM_011735203.2 Macaca fascicularis long-tailed macaque 102130864 XM_005593037.2 Cercocebus atys sooty mangabey 105574684 XM_012035809.1 Mandrillus leucophaeus drill 105550583 XM_011995533.1 Papio anubis olive baboon 101008749 XM_021933040.1 Theropithecus gelada gelada 112615413 XM_025372062.1 Chlorocebus sabaeus vervet 103231639 XM_007991113.1 Alouatta palliata mantled howler monkey N/A N/A unpublished draft genome, sequence in supplemental file Aotus nancymaae Ma’s night monkey 105705080 XM_012434682.2 Cebus capucinus imitator white-faced capuchin 108291904 XM_017512376.1 Sapajus apella tufted capuchin 116556688 XM_032285963.1 Saimiri boliviensis Bolivian squirrel monkey 101045190 XM_010336623.1 Callithrix jacchus common marmoset 100408882 XM_017968359.1 -

Evolutionary Stasis of the Pseudoautosomal Boundary in Strepsirrhine Primates
This is a repository copy of Evolutionary stasis of the pseudoautosomal boundary in strepsirrhine primates. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/168368/ Version: Published Version Article: Shearn, R., Wright, A.E., Mousset, S. et al. (7 more authors) (2020) Evolutionary stasis of the pseudoautosomal boundary in strepsirrhine primates. eLife, 9. e63650. ISSN 2050- 084X https://doi.org/10.7554/elife.63650 Reuse This article is distributed under the terms of the Creative Commons Attribution (CC BY) licence. This licence allows you to distribute, remix, tweak, and build upon the work, even commercially, as long as you credit the authors for the original work. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ SHORT REPORT Evolutionary stasis of the pseudoautosomal boundary in strepsirrhine primates Rylan Shearn1, Alison E Wright2, Sylvain Mousset1,3, Corinne Re´ gis1, Simon Penel1, Jean-Franc¸ois Lemaitre1, Guillaume Douay4, Brigitte Crouau-Roy5, Emilie Lecompte5, Gabriel AB Marais1,6* 1Laboratoire Biome´trie et Biologie Evolutive, CNRS / Univ. Lyon 1, Villeurbanne, France; 2Department of Animal and Plant Sciences, University of Sheffield, Sheffield, United Kingdom; 3Faculty of Mathematics, University of Vienna, Vienna, Austria; 4Zoo de Lyon, Lyon, France; 5Laboratoire Evolution et Diversite´ Biologique, CNRS / Univ. Toulouse, Toulouse, France; 6LEAF-Linking Landscape, Environment, Agriculture and Food Dept, Instituto Superior de Agronomia, Universidade de Lisboa, Lisbon, Portugal Abstract Sex chromosomes are typically comprised of a non-recombining region and a recombining pseudoautosomal region.