MINIREVIEW Methane from Acetate JAMES G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reduction by the Methylreductase System in Methanobacterium Bryantii WILLIAM B
JOURNAL OF BACTERIOLOGY, Jan. 1987, p. 87-92 Vol. 169, No. 1 0021-9193/87/010087-06$02.00/0 Copyright © 1987, American Society for Microbiology Inhibition by Corrins of the ATP-Dependent Activation and CO2 Reduction by the Methylreductase System in Methanobacterium bryantii WILLIAM B. WHITMAN'* AND RALPH S. WOLFE2 Department of Microbiology, University of Georgia, Athens, Georgia 30602,1 and Department of Microbiology, University ofIllinois, Urbana, Illinois 618012 Received 1 August 1986/Accepted 28 September 1986 Corrins inhibited the ATP-dependent activation of the methylreductase system and the methyl coenzyme M-dependent reduction of CO2 in extracts of Methanobacterium bryantii resolved from low-molecular-weight factors. The concentrations of cobinamides and cobamides required for one-half of maximal inhibition of the ATP-depen4ent activation were between 1 and 5 ,M. Cobinamides were more inhibitory at lower concentra- tiops than cobamides. Deoxyadenosylcobalamin was not inhibitory at concentrations up to 25 ,uM. The inhibition of CO2 reduction was competitive with respect to CO2. The concentration of methylcobalamin required for one-half of maximal inhibition was 5 ,M. Other cobamideg inhibited at similar concentrations, but diaquacobinami4e inhibited at lower concentrations. With respect to their affinities and specificities for corrins, inhibition of both the ATP-dependent activation'and CO2 reduction closely resembled the corrin- dependent activation of the methylreductase described in similar extracts (W. B. Whitman and R. S. Wolfe, J. Bacteriol. 164:165-172, 1985). However, whether the multiple effects of corrins are due to action at a single site is unknown. The effect of corrins (cobamides and cobinamides) on in CO2 reduction. -

The Vertical Distribution of Sediment Archaeal Community in the (Black Bloom) Disturbing Zhushan Bay of Lake Taihu
Hindawi Publishing Corporation Archaea Volume 2016, Article ID 8232135, 8 pages http://dx.doi.org/10.1155/2016/8232135 Research Article The Vertical Distribution of Sediment Archaeal Community in the (Black Bloom) Disturbing Zhushan Bay of Lake Taihu Xianfang Fan1,2 and Peng Xing1 1 State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography & Limnology, Chinese Academy of Sciences, Nanjing 210008, China 2State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China Correspondence should be addressed to Peng Xing; [email protected] Received 20 August 2015; Revised 27 November 2015; Accepted 20 December 2015 Academic Editor: William B. Whitman Copyright © 2016 X. Fan and P. Xing. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Using the Illumina sequencing technology, we investigated the vertical distribution of archaeal community in the sediment of Zhushan Bay of Lake Taihu, where the black bloom frequently occurred in summer. Overall, the Miscellaneous Crenarchaeotal Group (MCG), Deep Sea Hydrothermal Vent Group 6 (DHVEG-6), and Methanobacterium dominated the archaeal community. However, we observed significant difference in composition of archaeal community among different depths of the sediment. DHVEG-6 dominated in the surface layer (0–3 cm) sediment. Methanobacterium was the dominating archaeal taxa in the L2 (3– 6 cm) and L3 (6–10) sediment. MCG was most abundant in the L4 (10–15 cm) and L5 (15–20 cm) sediment. Besides, DHVEG-6 was significantly affected by the concentration of total phosphorus ).(TP And loss on ignition (LOI) was an important environmental factor for Methanobacterium. -

The Genome Characteristics and Predicted Function of Methyl-Group Oxidation Pathway in the Obligate Aceticlastic Methanogens, Methanosaeta Spp
The Genome Characteristics and Predicted Function of Methyl-Group Oxidation Pathway in the Obligate Aceticlastic Methanogens, Methanosaeta spp. Jinxing Zhu1,2, Huajun Zheng3, Guomin Ai1, Guishan Zhang1, Di Liu4, Xiaoli Liu1, Xiuzhu Dong1* 1 State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, Beijing, People’s Republic of China, 2 Graduate School, Chinese Academy of Sciences, Beijing, People’s Republic of China, 3 Shanghai-MOST Key Laboratory of Health and Disease Genomics, Chinese National Human Genome Center at Shanghai, Shanghai, People’s Republic of China, 4 Information center, Institute of Microbiology, Chinese Academy of Sciences, Beijing, People’s Republic of China Abstract In this work, we report the complete genome sequence of an obligate aceticlastic methanogen, Methanosaeta harundinacea 6Ac. Genome comparison indicated that the three cultured Methanosaeta spp., M. thermophila, M. concilii and M. harundinacea 6Ac, each carry an entire suite of genes encoding the proteins involved in the methyl-group oxidation pathway, a pathway whose function is not well documented in the obligately aceticlastic methanogens. Phylogenetic analysis showed that the methyl-group oxidation-involving proteins, Fwd, Mtd, Mch, and Mer from Methanosaeta strains cluster with the methylotrophic methanogens, and were not closely related to those from the hydrogenotrophic methanogens. Quantitative PCR detected the expression of all genes for this pathway, albeit ten times lower than the genes for aceticlastic methanogenesis in strain 6Ac. Western blots also revealed the expression of fwd and mch, genes involved in methyl-group oxidation. Moreover, 13C-labeling experiments suggested that the Methanosaeta strains might use the pathway as a methyl oxidation shunt during the aceticlastic metabolism. -
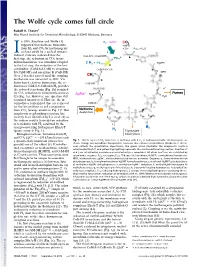
The Wolfe Cycle Comes Full Circle
The Wolfe cycle comes full circle Rudolf K. Thauer1 Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany n 1988, Rouvière and Wolfe (1) H - ΔμNa+ 2 CO2 suggested that methane formation + MFR from H and CO by methanogenic + 2H+ *Fd + H O I 2 2 ox 2 archaea could be a cyclical process. j O = Indirect evidence indicated that the CoB-SH + CoM-SH fi *Fd 2- a rst step, the reduction of CO2 to for- red R mylmethanofuran, was somehow coupled + * H MPT 2 H2 Fdox 4 to the last step, the reduction of the het- h erodisulfide (CoM-S-S-CoB) to coenzyme CoM-S-S-CoB b MFR M (CoM-SH) and coenzyme B (CoB-SH). H Over 2 decades passed until the coupling C 4 10 mechanism was unraveled in 2011: Via g flavin-based electron bifurcation, the re- CoB-SH duction of CoM-S-S-CoB with H provides 2 H+ the reduced ferredoxin (Fig. 1h) required c + Purines for CO2 reduction to formylmethanofuran ΔμNa + H MPT 4 f H O (2) (Fig. 1a). However, one question still 2 remained unanswered: How are the in- termediates replenished that are removed CoM-SH for the biosynthesis of cell components H Methionine d from CO2 (orange arrows in Fig. 1)? This Acetyl-CoA e anaplerotic (replenishing) reaction has F420 F420H2 recently been identified by Lie et al. (3) as F420 F420H2 the sodium motive force-driven reduction H i of ferredoxin with H2 catalyzed by the i energy-converting hydrogenase EhaA-T H2 (green arrow in Fig. -

THE MASS of L-PYRROLYSINE in METHYLAMINE METHYLTRANSFERASES and the ROLE of ITS IMINE BOND in CATALYSIS DISSERTATION Presented I
THE MASS OF L-PYRROLYSINE IN METHYLAMINE METHYLTRANSFERASES AND THE ROLE OF ITS IMINE BOND IN CATALYSIS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Graduate School of The Ohio State University by Jitesh Anthony Aloysius Soares, M.S. The Ohio State University 2008 Dissertation Committee: Dr. Joseph A. Krzycki, Advisor Approved by Dr. Charles J. Daniels Dr. Mark Morrison ______________________ Dr. F. Robert Tabita Advisor Graduate Program in Microbiology ABSTRACT Methanosarcina barkeri is an archaeon capable of producing methane from methylamines. Methylamine methyltransferases initiate methanogenesis from methylamines by transferring methyl groups to a cognate corrinoid protein. Each gene encoding a methylamine methyltransferase has been shown to contain a single in-frame amber codon. Further studies have shown that in the monomethylamine methyltransferase, mtmB , the amber codon encodes a novel amino acid, L-pyrrolysine. X-ray crystal structures of MtmB have shown that the structure of this amino acid is a lysine residue with the epsilon-nitrogen in amide linkage to a (4R, 5R)-4-substituted pyrrolyine-5-carboxylate ring. However, these structures did not allow an assignment of the pyrroline ring C4 substituent as a methyl or amine group. In this thesis (Chapter 2) mass spectrometry of chymotryptic digests of methylamine methyltransferases is employed to show that pyrrolysine in present in all three types of methylamine methyltransferase at the position corresponding to the amber codon in their respective genes. The mass of this amber-encoded residue was observed to coincide with the predicted mass of pyrrolysine with a methyl- group at the C4 position. -

Annotation Guidelines for Experimental Procedures
Annotation Guidelines for Experimental Procedures Developed By Mohammed Alliheedi Robert Mercer Version 1 April 14th, 2018 1- Introduction and background information What is rhetorical move? A rhetorical move can be defined as a text fragment that conveys a distinct communicative goal, in other words, a sentence that implies an author’s specific purpose to readers. What are the types of rhetorical moves? There are several types of rhetorical moves. However, we are interested in 4 rhetorical moves that are common in the method section of a scientific article that follows the Introduction Methods Results and Discussion (IMRaD) structure. 1- Description of a method: It is concerned with a sentence(s) that describes experimental events (e.g., “Beads with bound proteins were washed six times (for 10 min under rotation at 4°C) with pulldown buffer and proteins harvested in SDS-sample buffer, separated by SDS-PAGE, and analyzed by autoradiography.” (Ester & Uetz, 2008)). 2- Appeal to authority: It is concerned with a sentence(s) that discusses the use of standard methods, protocols, and procedures. There are two types of this move: - A reference to a well-established “standard” method (e.g., the use of a method like “PCR” or “electrophoresis”). - A reference to a method that was previously described in the literature (e.g., “Protein was determined using fluorescamine assay [41].” (Larsen, Frandesn and Treiman, 2001)). 3- Source of materials: It is concerned with a sentence(s) that lists the source of biological materials that are used in the experiment (e.g., “All microalgal strains used in this study are available at the Elizabeth Aidar Microalgae Culture Collection, Department of Marine Biology, Federal Fluminense University, Brazil.” (Larsen, Frandesn and Treiman, 2001)). -

Characteristics and Metabolic Patterns of Soil Methanogenic Archaea Communities in the High Latitude Natural Wetlands of China
Characteristics and Metabolic Patterns of Soil Methanogenic Archaea Communities in the High Latitude Natural Wetlands of China Di Wu Northeast Forestry University Caihong Zhao Northeast Forestry University Hui Bai Forestry Science Research Institute of Heilongjiang Province Fujuan Feng Northeast Forestry University Xin Sui Heilongjiang University Guangyu Sun ( [email protected] ) Northeast Forestry University Research article Keywords: Wetlands, Methanogens, Community diversity, Indicator species, Methanogenic metabolic patterns Posted Date: August 12th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-54821/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/18 Abstract Background: Soil methanogenic microorganisms are one of the primary methane-producing microbes in wetlands. However, we still poorly understand the community characteristic and metabolic patterns of these microorganisms according to vegetation type and seasonal changes. Therefore, to better elucidate the effects of the vegetation type and seasonal factors on the methanogenic community structure and metabolic patterns, we detected the characteristics of the soil methanogenic mcrA gene from three types of natural wetlands in different seasons in the Xiaoxing'an Mountain region, China. Result: The results indicated that the distribution of Methanobacteriaceae (hydrogenotrophic methanogens) was higher in winter, while Methanosarcinaceae and Methanosaetaceae accounted for a higher proportion in summer. Hydrogenotrophic methanogenesis was the dominant trophic pattern in each wetland. The results of principal coordinate analysis and cluster analysis showed that the vegetation type considerably inuenced the methanogenic community composition. The methanogenic community structure in the Betula platyphylla – Larix gmelinii wetland was relatively different from the structure of the other two wetland types. -

Untargetted Metabolomic Exploration of the Mycobacterium Tuberculosis Stress Response to Cinnamon Essential Oil
biomolecules Article Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil Elwira Sieniawska 1,* , Rafał Sawicki 2 , Joanna Golus 2 and Milen I. Georgiev 3,4 1 Chair and Department of Pharmacognosy, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland 2 Chair and Department of Biochemistry and Biotechnology, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland; [email protected] (R.S.); [email protected] (J.G.) 3 Group of Plant Cell Biotechnology and Metabolomics, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, 139 Ruski Blvd., 4000 Plovdiv, Bulgaria; [email protected] 4 Center of Plant Systems Biology and Biotechnology, 4000 Plovdiv, Bulgaria * Correspondence: [email protected] Received: 6 January 2020; Accepted: 24 February 2020; Published: 26 February 2020 Abstract: The antimycobacterial activity of cinnamaldehyde has already been proven for laboratory strains and for clinical isolates. What is more, cinnamaldehyde was shown to threaten the mycobacterial plasma membrane integrity and to activate the stress response system. Following promising applications of metabolomics in drug discovery and development we aimed to explore the mycobacteria response to cinnamaldehyde within cinnamon essential oil treatment by untargeted liquid chromatography–mass spectrometry. The use of predictive metabolite pathway analysis and description of produced lipids enabled the evaluation of the stress symptoms shown by bacteria. This study suggests that bacteria exposed to cinnamaldehyde could reorganize their outer membrane as a physical barrier against stress factors. They probably lowered cell wall permeability and inner membrane fluidity, and possibly redirected carbon flow to store energy in triacylglycerols. Being a reactive compound, cinnamaldehyde may also contribute to disturbances in bacteria redox homeostasis and detoxification mechanisms. -

Translation of the Amber Codon in Methylamine Methyltransferase Genes of a Methanogenic Archaeon
TRANSLATION OF THE AMBER CODON IN METHYLAMINE METHYLTRANSFERASE GENES OF A METHANOGENIC ARCHAEON DISSERTATION Presented in Partial Fulfillment of the Requirements for The Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Gayathri Srinivasan, M. S. * * * * * The Ohio State University 2003 Dissertation Committee: Dr. Joseph A. Krzycki, Advisor Approved by Dr. Charles J. Daniels Dr. Tina M. Henkin ________________________ (Advisor) Dr. John N. Reeve Department of Microbiology ABSTRACT Members of the Methanosarcinaceae family can in addition to hydrogen/carbon dioxide utilize several methylated compounds and convert them to methane. Methanogenesis from methylamines involves methylamine specific methyltransferases that transfer the methyl group from the methylamines to a corrinoid protein. The methylamine specific methyltransferase genes contain a single in-frame amber codon that is not read as a translational stop. The residue encoded by the amber codon, has been found to be a novel amino acid, pyrrolysine in MtmB. Multiple copies of monomethylamine methyltransferase genes (mtmB) containing a single amber codon within their open reading frames, along with the genes encoding their cognate corrinoid proteins (mtmC), exist within the genomes of the members of the Methanosarcinaceae family. The two copies of mtmCB genes from M. barkeri MS are differentially transcribed. Editing of the mtmB2 transcript was not detected suggesting a mechanism of amber codon readthrough occurring in the organism. Similar to selenocysteine incorporation at UGA codons, the Methanosarcinaceae also appear to have a unique mechanism for amber codon readthrough. An amber decoding tRNA gene, pylT, along with its cognate lysyl tRNA synthetase, pylS, are found near the MMA methyltransferase gene cluster. -

Xuejun Yu Dissertation Final
UNIVERSITY OF CALIFORNIA RIVERSIDE Conversion of Carbon Dioxide to Formate by a Formate Dehydrogenase from Cupriavidus necator A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Bioengineering by Xuejun Yu September 2018 Dissertation Committee: Dr. Ashok Mulchandani, Co-Chairperson Dr. Xin Ge, Co-Chairperson Dr. Russ Hille Copyright by Xuejun Yu 2018 The Dissertation of Xuejun Yu is approved: Committee Co-Chairperson Committee Co-Chairperson University of California, Riverside ACKNOWLEDGEMENTS I would like to express sincere appreciation to my advisor, Professor Ashok Mulchandani, for accepting me to be his student when I was suffering. Thank you very much for your delicate guidance, support and encouragement during the past years. You not only guide me on my PhD study, but also help me to be mature on my personality. From bottom of my heart, I feel very lucky to have you as professor. Also, many thanks go to my other committee members. Professor Xin Ge acted as my co-advisor and provided many valuable comments on my molecular cloning work. Professor Russ Hille has also provided insights, encouragements and advices as a member of my committee members. I have learned so many important insights from our meetings and discussions and thank you for bringing me into the molybdenum/tungsten enzyme conference. I am also thankful to the past and current group members, colleagues and friends - Dimitri Niks, Pankaj Ramnani, Feng Tan, Rabeay Hassan, Trupti Terse, Thien-Toan Tran, Claudia Chaves, Jia-wei Tay, Hui Wang, Pham Tung, Hilda Chan, Yingning Gao and Tynan Young. -

A Systematic Approach Re-Analyzing the Effects of Temperature
www.nature.com/scientificreports OPEN A systematic approach re-analyzing the efects of temperature disturbance on the microbial Received: 12 September 2018 Accepted: 12 April 2019 community of mesophilic anaerobic Published: xx xx xxxx digestion Grace Tzun-Wen Shaw , Chieh-Yin Weng, Cheng-Yu Chen , Francis Cheng-Hsuan Weng & Daryi Wang Microbial communities are key drivers of ecosystem processes, but their behavior in disturbed environments is difcult to measure. How microbial community composition and function respond disturbances is a common challenge in biomedical, environmental, agricultural, and bioenergy research. A novel way to solve this problem is to use a systems-level perspective and describe microbial communities as networks. Based on a mesophilic anaerobic digestion system of swine manure as a tool, we propose a simple framework to investigate changes in microbial communities via compositions, metabolic pathways, genomic properties and interspecies relationships in response to a long-term temperature disturbance. After temperature disturbance, microbial communities tend towards a competitive interaction network with higher GC content and larger genome size. Based on microbial interaction networks, communities responded to the disturbance by showing a transition from acetotrophic (Methanotrichaceae and Methanosarcinaceae) to methylotrophic methanogens (Methanomassiliicoccaceae and Methanobacteriaceae) and a fuctuation in rare biosphere taxa. To conclude, this study may be important for exploring the dynamic relationships between disturbance and microbial communities as a whole, as well as for providing researchers with a better understanding of how changes in microbial communities relate to ecological processes. Environmental disturbances are important factors in shaping the structure of plant, animal or microbial pop- ulations. In the past few decades, remarkable progress has been made towards understanding how disturbance afects microbial composition, biodiversity loss and ecological processes1–5. -

Characterization of Methanosarcina Barkeri MST and 227, Methanosarcina Mazei S-6T, and Methanosarcina Vacuolata Z-76IT GLORIA M
INTERNATIONALJOURNAL OF SYSTEMATICBACTERIOLOGY, Apr. 1991, p. 267-274 Vol. 41, No. 2 0020-7713/91/020267-08$02.OO/O Copyright 0 1991, International Union of Microbiological Societies Characterization of Methanosarcina barkeri MST and 227, Methanosarcina mazei S-6T, and Methanosarcina vacuolata Z-76IT GLORIA M. MAESTROJUAN' AND DAVID R. BOONE172* Departments of Environmental Science and Engineering' and Chemical and Biological Science,2 Oregon Graduate Institute, 19600 N.W. von Neumann Drive, Beaverton, Oregon 97006-1999 Members of the genus Methanosarcina are recognized as major aceticlastic methanogens, and several species which thrive in low-salt, pH-neutral culture medium at mesophilic temperatures have been described. However, the environmental conditions which support the fastest growth of these species (Methanosarcina barkeri MST [T = type strain] and 227, Methanosarcina mazei S-6T, and Methanosarcina vacuolata Z-761T) have not been reported previously. Although the members of the genus Methanosarcina are widely assumed to grow best at pH values near neutrality, we found that some strains prefer acidic pH values. M. vacuolata and the two strains of M. barkeri which we tested were acidophilic when they were grown on H, plus methanol, growing most rapidly at pH 5 and growing at pH values as low as 4.3. M. mazei grew best at pH values near neutrality. We found that all of the strains tested grew most rapidly at 37 to 42°C on all of the growth substrates which we tested. None of the strains was strongly halophilic, although the growth of some strains was slightly stimulated by small amounts of added NaCI.