From Freshwater in China
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development and Evaluation of Rrna Targeted in Situ Probes and Phylogenetic Relationships of Freshwater Fungi
Development and evaluation of rRNA targeted in situ probes and phylogenetic relationships of freshwater fungi vorgelegt von Diplom-Biologin Christiane Baschien aus Berlin Von der Fakultät III - Prozesswissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktorin der Naturwissenschaften - Dr. rer. nat. - genehmigte Dissertation Promotionsausschuss: Vorsitzender: Prof. Dr. sc. techn. Lutz-Günter Fleischer Berichter: Prof. Dr. rer. nat. Ulrich Szewzyk Berichter: Prof. Dr. rer. nat. Felix Bärlocher Berichter: Dr. habil. Werner Manz Tag der wissenschaftlichen Aussprache: 19.05.2003 Berlin 2003 D83 Table of contents INTRODUCTION ..................................................................................................................................... 1 MATERIAL AND METHODS .................................................................................................................. 8 1. Used organisms ............................................................................................................................. 8 2. Media, culture conditions, maintenance of cultures and harvest procedure.................................. 9 2.1. Culture media........................................................................................................................... 9 2.2. Culture conditions .................................................................................................................. 10 2.3. Maintenance of cultures.........................................................................................................10 -

Four New Freshwater Fungi Associated with Submerged Wood from Southwest Asia 191-203 Four New Freshwater Fungi Associated with Submerged Wood from Southwest Asia
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Sydowia Jahr/Year: 2010 Band/Volume: 062 Autor(en)/Author(s): Hu D. M., Cai Lei, Chen H., Bahkali Ali H., Hyde Kevin D. Artikel/Article: Four new freshwater fungi associated with submerged wood from Southwest Asia 191-203 Four new freshwater fungi associated with submerged wood from Southwest Asia D.M. Hu1, 2, L. Cai3*, H. Chen1, A.H. Bahkali4 & K. D. Hyde1,4,5* 1 International Fungal Research & Development Centre, The Research Institute of Resource Insects, Chinese Academy of Forestry, Bailongsi, Kunming 650224, P.R. China 2 School of Chemistry and Life Science, Gannan Normal University, Ganzhou 341000, P.R. China 3 Key Laboratory of Systematic Mycology & Lichenology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, P.R. China 4 Botany and Microbiology Department, College of Science, King Saud University, Riyadh, Saudi Arabia 5 School of Science, Mae Fah Luang University, Chiang Rai, Thailand Hu D.M., Cai L., Chen H., Bahkali A.H. & Hyde K.D. (2010) Four new freshwa- ter fungi associated with submerged wood from Southwest Asia. – Sydowia 62 (2): 191–203. One new teleomorphic and three new anamorphic ascomycetes from fresh wa- ter are introduced in this paper based on morphological characters. The new teleo- morphic ascomycete Ascominuta ovalispora sp. nov. was collected from the north of Thailand. The three new anamorphic ascomycetes Acrogenospora ellipsoidea sp. nov., Dictyosporium biseriale sp. nov. and Vanakripa menglensis sp. nov. were col- lected from Yunnan, China. Keys to species of Acrogenospora and Vanakripa are provided. -

Metabolites from Nematophagous Fungi and Nematicidal Natural Products from Fungi As an Alternative for Biological Control
Appl Microbiol Biotechnol (2016) 100:3799–3812 DOI 10.1007/s00253-015-7233-6 MINI-REVIEW Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: metabolites from nematophagous ascomycetes Thomas Degenkolb1 & Andreas Vilcinskas1,2 Received: 4 October 2015 /Revised: 29 November 2015 /Accepted: 2 December 2015 /Published online: 29 December 2015 # The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Plant-parasitic nematodes are estimated to cause Keywords Phytoparasitic nematodes . Nematicides . global annual losses of more than US$ 100 billion. The num- Oligosporon-type antibiotics . Nematophagous fungi . ber of registered nematicides has declined substantially over Secondary metabolites . Biocontrol the last 25 years due to concerns about their non-specific mechanisms of action and hence their potential toxicity and likelihood to cause environmental damage. Environmentally Introduction beneficial and inexpensive alternatives to chemicals, which do not affect vertebrates, crops, and other non-target organisms, Nematodes as economically important crop pests are therefore urgently required. Nematophagous fungi are nat- ural antagonists of nematode parasites, and these offer an eco- Among more than 26,000 known species of nematodes, 8000 physiological source of novel biocontrol strategies. In this first are parasites of vertebrates (Hugot et al. 2001), whereas 4100 section of a two-part review article, we discuss 83 nematicidal are parasites of plants, mostly soil-borne root pathogens and non-nematicidal primary and secondary metabolites (Nicol et al. 2011). Approximately 100 species in this latter found in nematophagous ascomycetes. Some of these sub- group are considered economically important phytoparasites stances exhibit nematicidal activities, namely oligosporon, of crops. -

Molecular Systematics of the Marine Dothideomycetes
available online at www.studiesinmycology.org StudieS in Mycology 64: 155–173. 2009. doi:10.3114/sim.2009.64.09 Molecular systematics of the marine Dothideomycetes S. Suetrong1, 2, C.L. Schoch3, J.W. Spatafora4, J. Kohlmeyer5, B. Volkmann-Kohlmeyer5, J. Sakayaroj2, S. Phongpaichit1, K. Tanaka6, K. Hirayama6 and E.B.G. Jones2* 1Department of Microbiology, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, 90112, Thailand; 2Bioresources Technology Unit, National Center for Genetic Engineering and Biotechnology (BIOTEC), 113 Thailand Science Park, Paholyothin Road, Khlong 1, Khlong Luang, Pathum Thani, 12120, Thailand; 3National Center for Biothechnology Information, National Library of Medicine, National Institutes of Health, 45 Center Drive, MSC 6510, Bethesda, Maryland 20892-6510, U.S.A.; 4Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon, 97331, U.S.A.; 5Institute of Marine Sciences, University of North Carolina at Chapel Hill, Morehead City, North Carolina 28557, U.S.A.; 6Faculty of Agriculture & Life Sciences, Hirosaki University, Bunkyo-cho 3, Hirosaki, Aomori 036-8561, Japan *Correspondence: E.B. Gareth Jones, [email protected] Abstract: Phylogenetic analyses of four nuclear genes, namely the large and small subunits of the nuclear ribosomal RNA, transcription elongation factor 1-alpha and the second largest RNA polymerase II subunit, established that the ecological group of marine bitunicate ascomycetes has representatives in the orders Capnodiales, Hysteriales, Jahnulales, Mytilinidiales, Patellariales and Pleosporales. Most of the fungi sequenced were intertidal mangrove taxa and belong to members of 12 families in the Pleosporales: Aigialaceae, Didymellaceae, Leptosphaeriaceae, Lenthitheciaceae, Lophiostomataceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae, Phaeosphaeriaceae, Pleosporaceae, Testudinaceae and Trematosphaeriaceae. Two new families are described: Aigialaceae and Morosphaeriaceae, and three new genera proposed: Halomassarina, Morosphaeria and Rimora. -

Phylogeny and Taxonomy of Obscure Genera of Microfungi
Persoonia 22, 2009: 139–161 www.persoonia.org RESEARCH ARTICLE doi:10.3767/003158509X461701 Phylogeny and taxonomy of obscure genera of microfungi P.W. Crous1, U. Braun2, M.J. Wingfield3, A.R. Wood4, H.D. Shin5, B.A. Summerell6, A.C. Alfenas7, C.J.R. Cumagun8, J.Z. Groenewald1 Key words Abstract The recently generated molecular phylogeny for the kingdom Fungi, on which a new classification scheme is based, still suffers from an under representation of numerous apparently asexual genera of microfungi. In an Brycekendrickomyces attempt to populate the Fungal Tree of Life, fresh samples of 10 obscure genera of hyphomycetes were collected. Chalastospora These fungi were subsequently established in culture, and subjected to DNA sequence analysis of the ITS and LSU Cyphellophora nrRNA genes to resolve species and generic questions related to these obscure genera. Brycekendrickomyces Dictyosporium (Herpotrichiellaceae) is introduced as a new genus similar to, but distinct from Haplographium and Lauriomyces. Edenia Chalastospora is shown to be a genus in the Pleosporales, with two new species, C. ellipsoidea and C. obclavata, phylogeny to which Alternaria malorum is added as an additional taxon under its oldest epithet, C. gossypii. Cyphellophora taxonomy eugeniae is newly described in Cyphellophora (Herpotrichiellaceae), and distinguished from other taxa in the genus. Thedgonia Dictyosporium is placed in the Pleosporales, with one new species, D. streliziae. The genus Edenia, which was Trochophora recently introduced for a sterile endophytic fungus isolated in Mexico, is shown to be a hyphomycete (Pleospo Verrucisporota rales) forming a pyronellea-like synanamorph in culture. Thedgonia is shown not to represent an anamorph of Vonarxia Mycosphaerella, but to belong to the Helotiales. -

MMA MASTERLIST - Sorted by Taxonomy
MMA MASTERLIST - Sorted by Taxonomy Sunday, December 10, 2017 Page 1 of 86 Amoebozoa Mycetomycota Protosteliomycetes Protosteliales Ceratiomyxaceae Ceratiomyxa fruticulosa Ceratiomyxa fruticulosa var. fruticulosa Ceratiomyxa fruticulosa var. poroides Ceratiomyxa sp. Mycetozoa Myxogastrea Incertae Sedis in Myxogastrea Liceaceae Licea minima Stemonitidaceae Brefeldia maxima Comatricha pulchella Comatricha sp. Comatricha typhoides Stemonitis axifera Stemonitis fusca Stemonitis sp. Stemonitis splendens Chromista Oomycota Incertae Sedis in Oomycota Peronosporales Peronosporaceae Plasmopara viticola Pythiaceae Pythium deBaryanum Oomycetes Saprolegniales Saprolegniaceae Saprolegnia sp. Peronosporea Albuginales Albuginaceae Albugo candida Fungus Ascomycota Ascomycetes Boliniales Boliniaceae Camarops petersii Capnodiales Capnodiaceae Scorias spongiosa Diaporthales Gnomoniaceae Cryptodiaporthe corni Sydowiellaceae Stegophora ulmea Valsaceae Cryphonectria parasitica Valsella nigroannulata Elaphomycetales Elaphomycetaceae Elaphomyces granulatus Elaphomyces sp. Erysiphales Erysiphaceae Erysiphe aggregata Erysiphe cichoracearum Erysiphe polygoni Microsphaera extensa Phyllactinia guttata Podosphaera clandestina Uncinula adunca Uncinula necator Hysteriales Hysteriaceae Glonium stellatum Leotiales Bulgariaceae Crinula caliciiformis Crinula sp. Mycocaliciales Mycocaliciaceae Phaeocalicium polyporaeum Peltigerales Collemataceae Leptogium cyanescens Lobariaceae Sticta fimbriata Nephromataceae Nephroma helveticum Peltigeraceae Peltigera evansiana Peltigera -

Supplementary Table S1 18Jan 2021
Supplementary Table S1. Accurate scientific names of plant pathogenic fungi and secondary barcodes. Below is a list of the most important plant pathogenic fungi including Oomycetes with their accurate scientific names and synonyms. These scientific names include the results of the change to one scientific name for fungi. For additional information including plant hosts and localities worldwide as well as references consult the USDA-ARS U.S. National Fungus Collections (http://nt.ars- grin.gov/fungaldatabases/). Secondary barcodes, where available, are listed in superscript between round parentheses after generic names. The secondary barcodes listed here do not represent all known available loci for a given genus. Always consult recent literature for which primers and loci are required to resolve your species of interest. Also keep in mind that not all barcodes are available for all species of a genus and that not all species/genera listed below are known from sequence data. GENERA AND SPECIES NAME AND SYNONYMYS DISEASE SECONDARY BARCODES1 Kingdom Fungi Ascomycota Dothideomycetes Asterinales Asterinaceae Thyrinula(CHS-1, TEF1, TUB2) Thyrinula eucalypti (Cooke & Massee) H.J. Swart 1988 Target spot or corky spot of Eucalyptus Leptostromella eucalypti Cooke & Massee 1891 Thyrinula eucalyptina Petr. & Syd. 1924 Target spot or corky spot of Eucalyptus Lembosiopsis eucalyptina Petr. & Syd. 1924 Aulographum eucalypti Cooke & Massee 1889 Aulographina eucalypti (Cooke & Massee) Arx & E. Müll. 1960 Lembosiopsis australiensis Hansf. 1954 Botryosphaeriales Botryosphaeriaceae Botryosphaeria(TEF1, TUB2) Botryosphaeria dothidea (Moug.) Ces. & De Not. 1863 Canker, stem blight, dieback, fruit rot on Fusicoccum Sphaeria dothidea Moug. 1823 diverse hosts Fusicoccum aesculi Corda 1829 Phyllosticta divergens Sacc. 1891 Sphaeria coronillae Desm. -

Australia Biodiversity of Biodiversity Taxonomy and and Taxonomy Plant Pathogenic Fungi Fungi Plant Pathogenic
Taxonomy and biodiversity of plant pathogenic fungi from Australia Yu Pei Tan 2019 Tan Pei Yu Australia and biodiversity of plant pathogenic fungi from Taxonomy Taxonomy and biodiversity of plant pathogenic fungi from Australia Australia Bipolaris Botryosphaeriaceae Yu Pei Tan Curvularia Diaporthe Taxonomy and biodiversity of plant pathogenic fungi from Australia Yu Pei Tan Yu Pei Tan Taxonomy and biodiversity of plant pathogenic fungi from Australia PhD thesis, Utrecht University, Utrecht, The Netherlands (2019) ISBN: 978-90-393-7126-8 Cover and invitation design: Ms Manon Verweij and Ms Yu Pei Tan Layout and design: Ms Manon Verweij Printing: Gildeprint The research described in this thesis was conducted at the Department of Agriculture and Fisheries, Ecosciences Precinct, 41 Boggo Road, Dutton Park, Queensland, 4102, Australia. Copyright © 2019 by Yu Pei Tan ([email protected]) All rights reserved. No parts of this thesis may be reproduced, stored in a retrieval system or transmitted in any other forms by any means, without the permission of the author, or when appropriate of the publisher of the represented published articles. Front and back cover: Spatial records of Bipolaris, Curvularia, Diaporthe and Botryosphaeriaceae across the continent of Australia, sourced from the Atlas of Living Australia (http://www.ala. org.au). Accessed 12 March 2019. Taxonomy and biodiversity of plant pathogenic fungi from Australia Taxonomie en biodiversiteit van plantpathogene schimmels van Australië (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit Utrecht op gezag van de rector magnificus, prof. dr. H.R.B.M. Kummeling, ingevolge het besluit van het college voor promoties in het openbaar te verdedigen op donderdag 9 mei 2019 des ochtends te 10.30 uur door Yu Pei Tan geboren op 16 december 1980 te Singapore, Singapore Promotor: Prof. -

Fungal Planet Description Sheets: 400–468
Persoonia 36, 2016: 316– 458 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158516X692185 Fungal Planet description sheets: 400–468 P.W. Crous1,2, M.J. Wingfield3, D.M. Richardson4, J.J. Le Roux4, D. Strasberg5, J. Edwards6, F. Roets7, V. Hubka8, P.W.J. Taylor9, M. Heykoop10, M.P. Martín11, G. Moreno10, D.A. Sutton12, N.P. Wiederhold12, C.W. Barnes13, J.R. Carlavilla10, J. Gené14, A. Giraldo1,2, V. Guarnaccia1, J. Guarro14, M. Hernández-Restrepo1,2, M. Kolařík15, J.L. Manjón10, I.G. Pascoe6, E.S. Popov16, M. Sandoval-Denis14, J.H.C. Woudenberg1, K. Acharya17, A.V. Alexandrova18, P. Alvarado19, R.N. Barbosa20, I.G. Baseia21, R.A. Blanchette22, T. Boekhout3, T.I. Burgess23, J.F. Cano-Lira14, A. Čmoková8, R.A. Dimitrov24, M.Yu. Dyakov18, M. Dueñas11, A.K. Dutta17, F. Esteve- Raventós10, A.G. Fedosova16, J. Fournier25, P. Gamboa26, D.E. Gouliamova27, T. Grebenc28, M. Groenewald1, B. Hanse29, G.E.St.J. Hardy23, B.W. Held22, Ž. Jurjević30, T. Kaewgrajang31, K.P.D. Latha32, L. Lombard1, J.J. Luangsa-ard33, P. Lysková34, N. Mallátová35, P. Manimohan32, A.N. Miller36, M. Mirabolfathy37, O.V. Morozova16, M. Obodai38, N.T. Oliveira20, M.E. Ordóñez39, E.C. Otto22, S. Paloi17, S.W. Peterson40, C. Phosri41, J. Roux3, W.A. Salazar 39, A. Sánchez10, G.A. Sarria42, H.-D. Shin43, B.D.B. Silva21, G.A. Silva20, M.Th. Smith1, C.M. Souza-Motta44, A.M. Stchigel14, M.M. Stoilova-Disheva27, M.A. Sulzbacher 45, M.T. Telleria11, C. Toapanta46, J.M. Traba47, N. -

2B0123fe4312dfc1e639b0d2192
Persoonia 20, 2008: 53–58 www.persoonia.org RESEARCH ARTICLE doi:10.3767/003158508X314732 Morphological and molecular characterisation of a new anamorphic genus Cheirosporium, from freshwater in China L. Cai1, X.Y. Guo2, K.D. Hyde3,4 Key words Abstract Cheirosporium gen. nov. is characterised by the production of sporodochial conidiomata, semimacrone matous to macronematous conidiophores that possess several distinct sterile branches, and cheiroid, smoothwalled ascomycetes conidia with rhexolytic secession. The 28S rDNA and ITS rDNA operon of this taxon were amplified and sequenced. Pleosporales A BLAST search revealed low homology between Cheirosporium triseriale and existing sequences in public data systematics bases, supporting the hypothesis that the species is new to science. Phylogenetic analysis showed that C. triseriale taxonomy groups with Dictyosporium and allied species, and nests within the Pleosporales (Dothideomycetes, Ascomycota). Cheirosporium is morphologically distinct from the cheirosporous genera Cheiromyces, Cheiromycina, Dictyosporium, Digitomyces, Digitodesmium and Pseudodictyosporium and these differences are discussed. Article info Received: 28 January 2008; Accepted: 17 April 2008; Published: 23 April 2008. INTRODUCTION ITS rDNA sequences showed that this fungus represents a new ascomycetous anamorph that could not be linked to a teleomor Freshwater fungi are taxonomically diverse, with more than phic genus. It is, therefore, described here as Cheirosporium 1 000 documented species, which include representatives from triseriale gen. & sp. nov. almost all major fungal classes (Tsui & Hyde 2003, Vijaykrishna et al. 2006). These fungi colonise many different substrates MATERIALS AND METHODS such as submerged plant litter, and aquatic organisms (Vijay krishna & Hyde 2006). Because most plant litter in freshwater is Submerged woody substrata were collected by Cai from a of terrestrial origin, the aquatic origin of most freshwater fungi is small stream in Yunnan, China. -
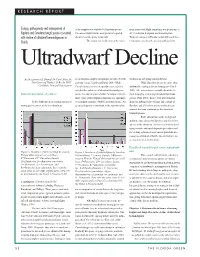
RESEARCH REPORT Ecology, Pathogenicity and Management Of
RESEARCH REPORT Ecology, pathogenicity and management of of the samples was attributed to Bipolaris species. from zoysia with blight symptoms, was pathogenic at Bipolaris and Curvularia fungal species associated Curvularia blight was the most prevalent reported 30° C in zoysia, bentgrass, and bermudagrass. with decline of ultradwarf bermudagrasses in disease from late spring to late fall. Turfgrass canopies in Florida consistently reach these Florida. The fungus was isolated off of the major- temperatures used for the previous pathogenicity Ultradwarf Decline By Dr. Lawrence E. Datnoff, Dr. Carol Stiles, Dr. ity of turfgrass samples brought into the labs (Stowell, studies from late spring through the fall. John Cisar and Matthew O. Brecht, Ph.D. personal comm.; Unruh and Davis, 2001). While While Bipolaris species are more often Candidate, Principal Investigators Curvularia species were frequently recovered from attributed to causing a disease in turfgrass (Couch both healthy and diseased ultradwarf bermudagrass 1995), little information is available about the role Rationale/description of problem: tissue, it is often unclear whether the fungus is the pri- these fungi play in affecting ultradwarf bermuda- mary cause of the turfgrass symptoms or a saprophyt- grasses (Pratt, 2001). In fact, very little is known In the Southeast, an increasing number of ic secondary organism (Stowell, personal comm.). An about the pathogenicity, etiology, and ecology of putting greens consist of the new ultradwarf accurate diagnosis is important to the superintendent Bipolaris and Curvularia species and no disease research has been conducted on the ultradwarf bermudagrasses. Basic information on the biology and ability to cause disease by Bipolaris and Curvularia species in the ultradwarf cultivars is critical for devel- oping accurate and rapid diagnostic procedures and for creating optimum, long-term integrated disease- management strategies that the superintendents can use to please their membership. -

Myconet Volume 14 Part One. Outine of Ascomycota – 2009 Part Two
(topsheet) Myconet Volume 14 Part One. Outine of Ascomycota – 2009 Part Two. Notes on ascomycete systematics. Nos. 4751 – 5113. Fieldiana, Botany H. Thorsten Lumbsch Dept. of Botany Field Museum 1400 S. Lake Shore Dr. Chicago, IL 60605 (312) 665-7881 fax: 312-665-7158 e-mail: [email protected] Sabine M. Huhndorf Dept. of Botany Field Museum 1400 S. Lake Shore Dr. Chicago, IL 60605 (312) 665-7855 fax: 312-665-7158 e-mail: [email protected] 1 (cover page) FIELDIANA Botany NEW SERIES NO 00 Myconet Volume 14 Part One. Outine of Ascomycota – 2009 Part Two. Notes on ascomycete systematics. Nos. 4751 – 5113 H. Thorsten Lumbsch Sabine M. Huhndorf [Date] Publication 0000 PUBLISHED BY THE FIELD MUSEUM OF NATURAL HISTORY 2 Table of Contents Abstract Part One. Outline of Ascomycota - 2009 Introduction Literature Cited Index to Ascomycota Subphylum Taphrinomycotina Class Neolectomycetes Class Pneumocystidomycetes Class Schizosaccharomycetes Class Taphrinomycetes Subphylum Saccharomycotina Class Saccharomycetes Subphylum Pezizomycotina Class Arthoniomycetes Class Dothideomycetes Subclass Dothideomycetidae Subclass Pleosporomycetidae Dothideomycetes incertae sedis: orders, families, genera Class Eurotiomycetes Subclass Chaetothyriomycetidae Subclass Eurotiomycetidae Subclass Mycocaliciomycetidae Class Geoglossomycetes Class Laboulbeniomycetes Class Lecanoromycetes Subclass Acarosporomycetidae Subclass Lecanoromycetidae Subclass Ostropomycetidae 3 Lecanoromycetes incertae sedis: orders, genera Class Leotiomycetes Leotiomycetes incertae sedis: families, genera Class Lichinomycetes Class Orbiliomycetes Class Pezizomycetes Class Sordariomycetes Subclass Hypocreomycetidae Subclass Sordariomycetidae Subclass Xylariomycetidae Sordariomycetes incertae sedis: orders, families, genera Pezizomycotina incertae sedis: orders, families Part Two. Notes on ascomycete systematics. Nos. 4751 – 5113 Introduction Literature Cited 4 Abstract Part One presents the current classification that includes all accepted genera and higher taxa above the generic level in the phylum Ascomycota.