Anterograde Amnesia and Temporally Graded Retrograde Amnesia for a Nonspatial Memory Task After Lesions of Hippocampus and Subiculum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Psychogenic and Organic Amnesia. a Multidimensional Assessment of Clinical, Neuroradiological, Neuropsychological and Psychopathological Features
Behavioural Neurology 18 (2007) 53–64 53 IOS Press Psychogenic and organic amnesia. A multidimensional assessment of clinical, neuroradiological, neuropsychological and psychopathological features Laura Serraa,∗, Lucia Faddaa,b, Ivana Buccionea, Carlo Caltagironea,b and Giovanni A. Carlesimoa,b aFondazione IRCCS Santa Lucia, Roma, Italy bClinica Neurologica, Universita` Tor Vergata, Roma, Italy Abstract. Psychogenic amnesia is a complex disorder characterised by a wide variety of symptoms. Consequently, in a number of cases it is difficult distinguish it from organic memory impairment. The present study reports a new case of global psychogenic amnesia compared with two patients with amnesia underlain by organic brain damage. Our aim was to identify features useful for distinguishing between psychogenic and organic forms of memory impairment. The findings show the usefulness of a multidimensional evaluation of clinical, neuroradiological, neuropsychological and psychopathological aspects, to provide convergent findings useful for differentiating the two forms of memory disorder. Keywords: Amnesia, psychogenic origin, organic origin 1. Introduction ness of the self – and a period of wandering. According to Kopelman [33], there are three main predisposing Psychogenic or dissociative amnesia (DSM-IV- factors for global psychogenic amnesia: i) a history of TR) [1] is a clinical syndrome characterised by a mem- transient, organic amnesia due to epilepsy [52], head ory disorder of nonorganic origin. Following Kopel- injury [4] or alcoholic blackouts [20]; ii) a history of man [31,33], psychogenic amnesia can either be sit- psychiatric disorders such as depressed mood, and iii) uation specific or global. Situation specific amnesia a severe precipitating stress, such as marital or emo- refers to memory loss for a particular incident or part tional discord [23], bereavement [49], financial prob- of an incident and can arise in a variety of circum- lems [23] or war [21,48]. -

DO AMNESICS EXHIBIT COGNITIVE DISSONANCE REDUCTION? the Role of Explicit Memory and Attention in Attitude Change Matthew D
PSYCHOLOGICAL SCIENCE Research Article DO AMNESICS EXHIBIT COGNITIVE DISSONANCE REDUCTION? The Role of Explicit Memory and Attention in Attitude Change Matthew D. Lieberman, Kevin N. Ochsner, Daniel T. Gilbert, and Daniel L. Schacter Harvard University Abstract—In two studies, we investigated the roles of explicit mem- CONSCIOUS CONTENT: THE ROLE OF ory and attentional resources in the process of behavior-induced atti- EXPLICIT MEMORY tude change. Although most theories of attitude change (cognitive Explicit memory refers to one’s ability to consciously recollect dissonance and self-perception theories) assume an important role for past events, behaviors, and experiences (Schacter, Chiu, & Ochsner, both mechanisms, we propose that behavior-induced attitude change 1993) and thus is a central component of most social abilities. Indeed, can be a relatively automatic process that does not require explicit people’s memory for the identities and actions of other people and memory for, or consciously controlled processing of, the discrepancy themselves forms the adhesive that gives them a continuing sense of between attitude and behavior. Using a free-choice paradigm, we place in their social world. Revising personal attitudes and beliefs in found that both amnesics and normal participants under cognitive response to a counterattitudinal behavior naturally seems to depend on load showed as much attitude change as did control participants. retrospective capacities. If Aesop’s forlorn lover of grapes could not remember that the grapes were out of reach, he would have no reason A fox saw some ripe black grapes hanging from a trellised vine. He resorted to to persuade himself of their reduced quality. -

Does Amnesia Specifically Predict Alzheimer's Pathology?
Does amnesia specifically predict Alzheimer’s pathology? A neuropathological study Maxime Bertoux, Pascaline Cassagnaud, Thibaud Lebouvier, Florence Lebert, Marie Sarazin, Isabelle Le Ber, Bruno Dubois, Brain Bank, Sophie Auriacombe, Didier Hannequin, et al. To cite this version: Maxime Bertoux, Pascaline Cassagnaud, Thibaud Lebouvier, Florence Lebert, Marie Sarazin, et al.. Does amnesia specifically predict Alzheimer’s pathology? A neuropathological study. Neurobiology of Aging, Elsevier, In press. hal-02898941 HAL Id: hal-02898941 https://hal.archives-ouvertes.fr/hal-02898941 Submitted on 14 Jul 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Amnesia/AD pathology 1 Does amnesia specifically predict Alzheimer’s pathology? A neuropathological study. Maxime Bertoux*1a, Pascaline Cassagnaud*b, Thibaud Lebouvier*c, Florence Leberta, Marie Sarazinde, Isabelle Le Berfg, Bruno Duboisfg, NeuroCEB Brain Bank, Sophie Auriacombeh, Didier Hannequini, David Walloni, Mathieu Ceccaldij, Claude-Alain Mauragek, Vincent Deramecourtc, Florence Pasquiera a Univ Lille, Lille Neuroscience & Cognition (Inserm UMRS1172) Degenerative and vascular cognitive disorders, CHU Lille, Laboratory of Excellence Distalz (Development of Innovative Strategies for a Transdisciplinary approach to ALZheimer’s disease). F-59000, Lille, France.F-59000, Lille, France. b Univ Lille, CHU Lille, Laboratory of Excellence Distalz (Development of Innovative Strategies for a Transdisciplinary approach to ALZheimer’s disease). -

When the Mind Falters: Cognitive Losses in Dementia
T L C When the Mind Falters: Cognitive Losses in Dementia by L Joel Streim, MD T Associate Professor of Psychiatry C Director, Geriatric Psychiatry Fellowship Program University of Pennsylvania VISN 4 Mental Illness Research Education and Clinical Center Philadelphia VA Medical Center Delaware Valley Geriatric Education Center The goal of this module is to teach direct staff about the syndrome of dementia and its clinical effects on residents. It focuses on the ways that the symptoms of dementia affect persons’ functional ability and behavior. We begin with an overview of the symptoms of cognitive impairment. We continue with a description of the causes, epidemiology, and clinical course (stages) of dementia. We then turn to a closer look at the specific areas of cognitive impairment, and examine how deficits in different areas of cognitive function can interfere with the person’s daily functioning, causing disability. The accompanying videotape illustrates these principles, using the example of a nursing home resident whose cognitive impairment interferes in various ways with her eating behavior and ability to feed herself. 1 T L Objectives C At the end of this module you should be able to: Describe the stages of dementia Distinguish among specific cognitive impairments from dementia L Link specific cognitive impairments with the T disabilities they cause C Give examples of cognitive impairments and disabilities Describe what to do when there is an acute change in cognitive or functional status Delaware Valley Geriatric Education Center At the end of this module you should be able to • Describe the stages of dementia. These are early, middle and late, and we discuss them in more detail. -

PAVOL JOZEF ŠAFARIK UNIVERSITY in KOŠICE Dissociative Amnesia: a Clinical and Theoretical Reconsideration DEGREE THESIS
PAVOL JOZEF ŠAFARIK UNIVERSITY IN KOŠICE FACULTY OF MEDICINE Dissociative amnesia: a clinical and theoretical reconsideration Paulo Alexandre Rocha Simão DEGREE THESIS Košice 2017 PAVOL JOZEF ŠAFARIK UNIVERSITY IN KOŠICE FACULTY OF MEDICINE FIRST DEPARTMENT OF PSYCHIATRY Dissociative amnesia: a clinical and theoretical reconsideration Paulo Alexandre Rocha Simão DEGREE THESIS Thesis supervisor: Mgr. MUDr. Jozef Dragašek, PhD., MHA Košice 2017 Analytical sheet Author Paulo Alexandre Rocha Simão Thesis title Dissociative amnesia: a clinical and theoretical reconsideration Language of the thesis English Type of thesis Degree thesis Number of pages 89 Academic degree M.D. University Pavol Jozef Šafárik University in Košice Faculty Faculty of Medicine Department/Institute Department of Psychiatry Study branch General Medicine Study programme General Medicine City Košice Thesis supervisor Mgr. MUDr. Jozef Dragašek, PhD., MHA Date of submission 06/2017 Date of defence 09/2017 Key words Dissociative amnesia, dissociative fugue, dissociative identity disorder Thesis title in the Disociatívna amnézia: klinické a teoretické prehodnotenie Slovak language Key words in the Disociatívna amnézia, disociatívna fuga, disociatívna porucha identity Slovak language Abstract in the English language Dissociative amnesia is a one of the most intriguing, misdiagnosed conditions in the psychiatric world. Dissociative amnesia is related to other dissociative disorders, such as dissociative identity disorder and dissociative fugue. Its clinical features are known -

The Three Amnesias
The Three Amnesias Russell M. Bauer, Ph.D. Department of Clinical and Health Psychology College of Public Health and Health Professions Evelyn F. and William L. McKnight Brain Institute University of Florida PO Box 100165 HSC Gainesville, FL 32610-0165 USA Bauer, R.M. (in press). The Three Amnesias. In J. Morgan and J.E. Ricker (Eds.), Textbook of Clinical Neuropsychology. Philadelphia: Taylor & Francis/Psychology Press. The Three Amnesias - 2 During the past five decades, our understanding of memory and its disorders has increased dramatically. In 1950, very little was known about the localization of brain lesions causing amnesia. Despite a few clues in earlier literature, it came as a complete surprise in the early 1950’s that bilateral medial temporal resection caused amnesia. The importance of the thalamus in memory was hardly suspected until the 1970’s and the basal forebrain was an area virtually unknown to clinicians before the 1980’s. An animal model of the amnesic syndrome was not developed until the 1970’s. The famous case of Henry M. (H.M.), published by Scoville and Milner (1957), marked the beginning of what has been called the “golden age of memory”. Since that time, experimental analyses of amnesic patients, coupled with meticulous clinical description, pathological analysis, and, more recently, structural and functional imaging, has led to a clearer understanding of the nature and characteristics of the human amnesic syndrome. The amnesic syndrome does not affect all kinds of memory, and, conversely, memory disordered patients without full-blown amnesia (e.g., patients with frontal lesions) may have impairment in those cognitive processes that normally support remembering. -

A Neurostructural Biomarker of Dissociative Amnesia: a Hippocampal Study in Dissociative Cambridge.Org/Psm Identity Disorder
Psychological Medicine A neurostructural biomarker of dissociative amnesia: a hippocampal study in dissociative cambridge.org/psm identity disorder 1,2, 3, 4,5,† Original Article Lora I. Dimitrova * , Sophie L. Dean *, Yolanda R. Schlumpf , Eline M. Vissia6,† , Ellert R. S. Nijenhuis5, Vasiliki Chatzi7, Lutz Jäncke4,8, *These authors contributed equally to this 2 9 1 work Dick J. Veltman , Sima Chalavi and Antje A. T. S. Reinders † These authors contributed equally to this 1Department of Psychological Medicine, Institute of Psychiatry, Psychology & Neuroscience, King’s College work London, London, UK; 2Department of Psychiatry, Amsterdam UMC, Location VUmc, VU University Amsterdam, Amsterdam, The Netherlands; 3Department of Psychosis Studies, Institute of Psychiatry, King’s College London, Cite this article: Dimitrova LI et al (2021). A 4 neurostructural biomarker of dissociative London, UK; Division of Neuropsychology, Department of Psychology, University of Zurich, Zurich, Switzerland; 5 6 amnesia: a hippocampal study in dissociative Clienia Littenheid AG, Private Clinic for Psychiatry and Psychotherapy, Littenheid, Switzerland; Heelzorg, Centre 7 identity disorder. Psychological Medicine 1–9. for Psychotrauma, Zwolle, The Netherlands; Department of Biomedical Engineering, King’s College London, https://doi.org/10.1017/S0033291721002154 London, UK; 8Research Unit for Plasticity and Learning of the Healthy Aging Brain, University of Zurich, Zurich, Switzerland and 9Movement Control and Neuroplasticity Research Group, Department of Movement Sciences, KU Received: 14 September 2020 Leuven, Leuven, Belgium Revised: 12 February 2021 Accepted: 11 May 2021 Abstract Key words: Background. Little is known about the neural correlates of dissociative amnesia, a transdiag- Dissociative experience scale; DES; childhood trauma; CA1; Freesurfer; dissociation nostic symptom mostly present in the dissociative disorders and core characteristic of dis- sociative identity disorder (DID). -

Neurodiversity 10Th Annual Nurturing Developing Minds Conference
Neurodiversity 10th Annual Nurturing Developing Minds Conference Manuel F. Casanova, M.D. SmartState Endowed Chair in Childhood Neurotherapeutics University of South Carolina Greenville Health System Conflict of Interests Neuronetics (TMS platform), Neuronetrix Incorporated, Clearly Present Foundation Pfizer, Eisai, Nycomed Amersham, Aventis Pasteur Limited, Medvantis Medical Service Council of Health Care Advisors for the Gerson Lehrman Goup Royalties: Springer, Nova, Taylor and Francis, John Wiley I am a physician who deals with individuals with neurodevelopmental disabilities and have a grandson with autism. Neurodiversity “A new wave of activists wants to celebrate atypical brain function as a positive identity, not a disability.” New York News and Politics “…neurological (brain wiring) differences, traditionally seen as disadvantages, are really advantages.” Fox and Hounds “What is autism: a devastating developmental disorder, a lifelong disability, or a naturally occurring form of cognitive difference akin to certain forms of genius?” SUPOZA.COM NEURODIVERSITY AND AUTISTIC PRIDE Individual with autism vs. Autistic Individual Control subject vs. Typically developing(TD) subject What message are you sending??? “Why is it that what makes me me, needs to be classified as a disability?” A child under 18 will be considered disabled if he or she has a medically determinable physical or mental impairment or combination of impairments that cause marked and severe functional limitations, that can be expected to cause death or that has lasted or can be expected to last for a continuous period of not less than 12 months. Normal variation in the human genome A social category rather than a medical disorder Includes autism, bipolar disorders, and other neurotypes It does not need to be cured ABA is specially pernicioius. -

Dissociative Disorders Types of Dissociative Disorders Dissociative
Dissociative Disorders • Similar to somatoform in some ways • Often not that concerned about memory loss • Often can be seen as form of escape Types of Dissociative Disorders • Depersonalization Disorder • Dissociative Amnesia (Generalized vs. Selective). • Dissociative Fugue • Dissociative Trance Disorder • Dissociative Identity Disorder (formerly Multiple Personality Disorder). Dissociative Disorders Involves sudden and temporary alteration in functions of consciousness Avoids stress and gratifies needs in manner allowing person to deny personal responsibility Escapes from core personality and personality processes Quite rare Dissociative Disorders Dissociative Disorders are typified by alterations in sense of self and reality Characteristic features include a sense of depersonalization or derealization. Dissociative Disorders Depersonalization is when one’s sense of your own reality is altered (your own personality and sense of self may be fragmented). Derealization is best described as when your sense of reality of the external world is altered. The external world feels unreal and unfamiliar Depersonalization •Feelings of detachment or estrangement •External world is perceived as unreal •May have : • Sensory anesthesia • Lack of affective response Depersonalization Characteristics • Feelings that you're an outside observer of your thoughts, feelings, your body or parts of your body —as if you were floating in air above yourself • Feeling like a robot or that you're not in control of your speech or movements • The sense that your -

COGNITIVE REHABILITATION in a Severely Brain-Injured PATIENT with Amnesia, TETRAPLEGIA and ANARTHRIA
J Rehabil Med 2009; 41: 393–396 CASE REPORT COMMUNICATING USING THE EYES WITHOUT REMEMBERING IT: COGNITIVE REHABILITATION IN A severely BRAIN-INJURED PATIENT WITH AMNESIA, TETRAPLEGIA AND ANARTHRIA Luigi Trojano, MD1,2, Pasquale Moretta, PsyD2 and Anna Estraneo, MD2 From the 1Neuropsychology Laboratory, Department of Psychology, Second University of Naples, Caserta and 2Salvatore Maugeri Foundation, IRCCS, Scientific Institute of Telese Terme (BN), Telese Terme, Italy We describe here a case of cognitive rehabilitation in a young LIS (4), but they nonetheless experience extreme difficulties patient with closed head injury, who had dense anterograde in communicating. For this reason patients with LIS can ac- amnesia and such disabling neurological defects (tetraplegia tively interact with the environment only by means of complex and anarthria) that the condition evoked some features of communicative systems, based for instance on brain-computer an incomplete locked-in syndrome. After a prolonged period interface devices (6). of no communicative possibility, the patient underwent a Patients with TBI with extremely severe motor disturbances specific training, based on principles of errorless learning, (tetraplegia and anarthria) face the same difficulties as patients with the aim of using a computerized eye-tracker system. with complete or incomplete LIS, but, in contrast to patients Although, due to memory disturbances, the patient always with LIS, they show the typical cognitive defects observed after denied ever having used the eye-tracker system, learned to a TBI. Therefore, any rehabilitative treatment in such patients use the computerized device and improved interaction with will be difficult and discouraging, even when it is aimed only the environment. -
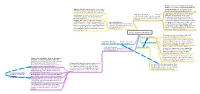
Long Term Memory & Amnesia
Previous theories of Amnesia: encoding faliure (consolidation) rapid forgetting and Amnesic Patients typically retain old procedural retrieval failure (retroactive/proactive information and are reasonably good at learning interference, Underwood; McGeoch) new procedural skills (H.M and mirror writing) Important Current Theory: Contextual Amnesia & the Brain Linked to Conditioning: eyeblink with puf of air leads to Memory Theory (Ryan et al. 2000) medial temporal lobe and diencephalic conditioned response. Patients with amnesia impairment in integrating of binding region, including mamillary bodies and typically acquire this. contextual/relational features of memory. thalamus. Priming: Improvement or bias in performance The medial temporal lobe and hippocampus resulting from prior, supraliminal presentation of Procedural Memory are proposed to bind events to the contexts stimuli. Tulving et al. (1982) primed participants Often relatively automatic processes in which they occur. This bypasses the with a list of words and then showed them letters (requiring little attention) allowing for declarative vs procedural distinction. And (cued-recall). Recall was better for words behavioural responses to there is reasonable support for this theory presented earlier. Amnesic patients are relatively environmental cues. (Channon, Shanks et al 2006.) normal on these tasks. Long-Term Memory & Amnesia Temporary storage of information with rapid Short-Term Memory decay and sensitivity to interference. Supported by studies of recency efect (Murdock, Postman), which is taken as evidence of short Long-Term Memory Mediates declarative Double Dissociation?! but not non-declarative (procedural) memory. term memory store. Also by studies supporting subvocal speech (Baddeley, 1966) However, studies have provided evidence of Declarative Memory This is knowledge STM link with LTM retrieved by explicit, deliberate recollection. -

Sustained Experience of Emotion After Loss of Memory in Patients with Amnesia
Sustained experience of emotion after loss of memory in patients with amnesia Justin S. Feinsteina,b,1, Melissa C. Duffa,c, and Daniel Tranela,b aDivision of Cognitive Neuroscience, Department of Neurology, University of Iowa College of Medicine, bDepartment of Psychology, and cDepartment of Communication Sciences and Disorders, University of Iowa, Iowa City, IA 52242-1053 Edited* by Marcus E. Raichle, Washington University, St. Louis, MO, and approved March 17, 2010 (received for review December 9, 2009) Can the experience of an emotion persist once the memory for what central feature of these patients’ condition is a profound induced the emotion has been forgotten? We capitalized on a rare impairment in forming new conscious memories about events that opportunity to study this question directly using a select group of unfold in their daily lives. This severe memory deficit confers a patients with severe amnesia following circumscribed bilateral unique opportunity to investigate whether the experience of an damage to the hippocampus. The amnesic patients underwent a emotion can outlast the memory for what caused it. sadness induction procedure (using affectively-laden film clips) to The dissociation between emotion and memory in patients with ascertain whether their experience of sadness would persist beyond amnesia harkens back to 1911, when the Swiss neurologist Cla- their memory for the sadness-inducing films. The experimentshowed parède concealed a pin between his fingers while greeting one of his that the patients continued to experience elevated levels of sadness amnesic patients with a handshake (18). The sharp pin surprised the well beyond the point in time at which they had lost factual memory patient and elicited a small amount of pain that quickly dissipated.