Convection, Conduction & Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Soaring Weather
Chapter 16 SOARING WEATHER While horse racing may be the "Sport of Kings," of the craft depends on the weather and the skill soaring may be considered the "King of Sports." of the pilot. Forward thrust comes from gliding Soaring bears the relationship to flying that sailing downward relative to the air the same as thrust bears to power boating. Soaring has made notable is developed in a power-off glide by a conven contributions to meteorology. For example, soar tional aircraft. Therefore, to gain or maintain ing pilots have probed thunderstorms and moun altitude, the soaring pilot must rely on upward tain waves with findings that have made flying motion of the air. safer for all pilots. However, soaring is primarily To a sailplane pilot, "lift" means the rate of recreational. climb he can achieve in an up-current, while "sink" A sailplane must have auxiliary power to be denotes his rate of descent in a downdraft or in come airborne such as a winch, a ground tow, or neutral air. "Zero sink" means that upward cur a tow by a powered aircraft. Once the sailcraft is rents are just strong enough to enable him to hold airborne and the tow cable released, performance altitude but not to climb. Sailplanes are highly 171 r efficient machines; a sink rate of a mere 2 feet per second. There is no point in trying to soar until second provides an airspeed of about 40 knots, and weather conditions favor vertical speeds greater a sink rate of 6 feet per second gives an airspeed than the minimum sink rate of the aircraft. -

Effect of Wind on Near-Shore Breaking Waves
EFFECT OF WIND ON NEAR-SHORE BREAKING WAVES by Faydra Schaffer A Thesis Submitted to the Faculty of The College of Engineering and Computer Science in Partial Fulfillment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, Florida December 2010 Copyright by Faydra Schaffer 2010 ii ACKNOWLEDGEMENTS The author wishes to thank her mother and family for their love and encouragement to go to college and be able to have the opportunity to work on this project. The author is grateful to her advisor for sponsoring her work on this project and helping her to earn a master’s degree. iv ABSTRACT Author: Faydra Schaffer Title: Effect of wind on near-shore breaking waves Institution: Florida Atlantic University Thesis Advisor: Dr. Manhar Dhanak Degree: Master of Science Year: 2010 The aim of this project is to identify the effect of wind on near-shore breaking waves. A breaking wave was created using a simulated beach slope configuration. Testing was done on two different beach slope configurations. The effect of offshore winds of varying speeds was considered. Waves of various frequencies and heights were considered. A parametric study was carried out. The experiments took place in the Hydrodynamics lab at FAU Boca Raton campus. The experimental data validates the knowledge we currently know about breaking waves. Offshore winds effect is known to increase the breaking height of a plunging wave, while also decreasing the breaking water depth, causing the wave to break further inland. Offshore winds cause spilling waves to react more like plunging waves, therefore increasing the height of the spilling wave while consequently decreasing the breaking water depth. -

NWS Unified Surface Analysis Manual
Unified Surface Analysis Manual Weather Prediction Center Ocean Prediction Center National Hurricane Center Honolulu Forecast Office November 21, 2013 Table of Contents Chapter 1: Surface Analysis – Its History at the Analysis Centers…………….3 Chapter 2: Datasets available for creation of the Unified Analysis………...…..5 Chapter 3: The Unified Surface Analysis and related features.……….……….19 Chapter 4: Creation/Merging of the Unified Surface Analysis………….……..24 Chapter 5: Bibliography………………………………………………….…….30 Appendix A: Unified Graphics Legend showing Ocean Center symbols.….…33 2 Chapter 1: Surface Analysis – Its History at the Analysis Centers 1. INTRODUCTION Since 1942, surface analyses produced by several different offices within the U.S. Weather Bureau (USWB) and the National Oceanic and Atmospheric Administration’s (NOAA’s) National Weather Service (NWS) were generally based on the Norwegian Cyclone Model (Bjerknes 1919) over land, and in recent decades, the Shapiro-Keyser Model over the mid-latitudes of the ocean. The graphic below shows a typical evolution according to both models of cyclone development. Conceptual models of cyclone evolution showing lower-tropospheric (e.g., 850-hPa) geopotential height and fronts (top), and lower-tropospheric potential temperature (bottom). (a) Norwegian cyclone model: (I) incipient frontal cyclone, (II) and (III) narrowing warm sector, (IV) occlusion; (b) Shapiro–Keyser cyclone model: (I) incipient frontal cyclone, (II) frontal fracture, (III) frontal T-bone and bent-back front, (IV) frontal T-bone and warm seclusion. Panel (b) is adapted from Shapiro and Keyser (1990) , their FIG. 10.27 ) to enhance the zonal elongation of the cyclone and fronts and to reflect the continued existence of the frontal T-bone in stage IV. -
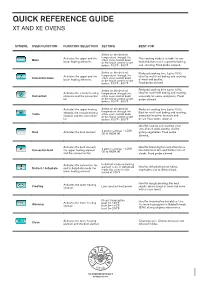
QUICK REFERENCE GUIDE XT and XE Ovens
QUICK REFERENCE GUIDE XT AND XE OVENS SYMBOL OVEN FUNCTION FUNCTION SELECTION SETTING BEST FOR Select on the desired Activates the upper and the temperature through the This cooking mode is suitable for any Bake either oven control knob lower heating elements or the touch control smart kind of dishes and it is great for baking botton. 100°F - 500°F and roasting. Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the upper and the temperature through the Ideal for multi-level baking and roasting Convection bake either oven control knob lower heating elements or the touch control smart of meat and poultry. botton. 100°F - 500°F Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the circular heating temperature through the Ideal for multi-level baking and roasting, Convection elements and the convection either oven control knob especially for cakes and pastry. Food fan or the touch control smart probe allowed. botton. 100°F - 500°F Activates the upper heating Select on the desired Reduced cooking time (up to 10%). temperature through the Best for multi-level baking and roasting, Turbo element, the circular heating either oven control knob element and the convection or the touch control smart especially for pizza, focaccia and fan botton. 100°F - 500°F bread. Food probe allowed. Ideal for searing and roasting small cuts of beef, pork, poultry, and for 4 power settings – LOW Broil Activates the broil element (1) to HIGH (4) grilling vegetables. Food probe allowed. Activates the broil element, Ideal for browning fish and other items 4 power settings – LOW too delicate to turn and thicker cuts of Convection broil the upper heating element (1) to HIGH (4) and the convection fan steaks. -

A STUDY of ELEVATED CONVECTION and ITS IMPACTS on SURFACE WEATHER CONDITIONS a Dissertat
A STUDY OF ELEVATED CONVECTION AND ITS IMPACTS ON SURFACE WEATHER CONDITIONS _______________________________________ A Dissertation presented to the Faculty of the Graduate School at the University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy _____________________________________________________ by Joshua Kastman Dr. Patrick Market, Dissertation Supervisor December 2017 © copyright by Joshua S. Kastman 2017 All Rights Reserved The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled A Study of Elevated Convection and its Impacts on Surface Weather Conditions presented by Joshua Kastman, a candidate for the degree of doctor of philosophy, Soil, Environmental, and Atmospheric Sciences and hereby certify that, in their opinion, it is worthy of acceptance. ____________________________________________ Professor Patrick Market ____________________________________________ Associate Professor Neil Fox ____________________________________________ Professor Anthony Lupo ____________________________________________ Associate Professor Sonja Wilhelm Stannis ACKNOWLEDGMENTS I would like to begin by thanking Dr. Patrick Market for all of his guidance and encouragement throughout my time at the University of Missouri. His advice has been invaluable during my graduate studies. His mentorship has meant so much to me and I look forward his advice and friendship in the years to come. I would also like to thank Anthony Lupo, Neil Fox and Sonja Wilhelm-Stannis for serving as committee members and for their advice and guidance. I would like to thank the National Science Foundation for funding the project. I would l also like to thank my wife Anna for her, support, patience and understanding while I pursued this degree. Her love and partnerships means everything to me. -

Oven Settings Lighting the Burners Plates Which Are Designed to Catch Drippings and All Burners Are Ignited by Electric Circulate a Smoke Flavor Back Into the Food
Surface Operation Range Controls Oven Settings Lighting the Burners plates which are designed to catch drippings and All burners are ignited by electric circulate a smoke flavor back into the food. Beneath the Interior Oven Left Front Burner Left Oven Left Oven Griddle Self-Clean Right Oven Right Front Burner BAKE (Two- require gentle cooking such as pastries, souffles, yeast MED BROIL ignition. There are no open-flame, flavor generator plates is a two piece drip pan which Light Switch Control Knob Function Temperature Indicator Light Indicator Temperature Control Knob Element Bake) breads, quick breads and cakes. Breads, cookies, and other Inner and outer broil “standing” pilots. catches any drippings that might pass beyond the flavor Full power heat is baked goods come out evenly textured with golden crusts. elements pulse on (15,000 BTU) Selector Knob Control Knob Light Indicator Light (15,000 BTU) generator plates. This unique grilling system is designed radiated from the No special bakeware is required. Use this function for single and off to produce VariSimmer™ to provide outdoor quality grilling indoors. bake element in the rack baking, multiple rack baking, roasting, and preparation less heat for slow Simmering is a cooking technique in CLEAN OVEN GRIDDLE OVEN CLEAN bottom of the oven of complete meals. This setting is also recommended when broiling. Allow about which foods are cooked in hot liquids kept at or just Dual Fuel cavity and baking large quantities of baked goods at one time. 4 inches (10 cm) Oven Functions Convection-Self Clean barely below the boiling point of water. -

FORECASTERS' FORUM Elevated Convection And
1280 WEATHER AND FORECASTING VOLUME 23 FORECASTERS’ FORUM Elevated Convection and Castellanus: Ambiguities, Significance, and Questions STEPHEN F. CORFIDI NOAA/NWS/NCEP/Storm Prediction Center, Norman, Oklahoma SARAH J. CORFIDI NOAA/NWS/NCEP/Storm Prediction Center, and Cooperative Institute for Mesoscale Meteorological Studies, University of Oklahoma, Norman, Oklahoma DAVID M. SCHULTZ* Cooperative Institute for Mesoscale Meteorological Studies, University of Oklahoma, and NOAA/National Severe Storms Laboratory, Norman, Oklahoma (Manuscript received 23 January 2008, in final form 26 April 2008) ABSTRACT The term elevated convection is used to describe convection where the constituent air parcels originate from a layer above the planetary boundary layer. Because elevated convection can produce severe hail, damaging surface wind, and excessive rainfall in places well removed from strong surface-based instability, situations with elevated storms can be challenging for forecasters. Furthermore, determining the source of air parcels in a given convective cloud using a proximity sounding to ascertain whether the cloud is elevated or surface based would appear to be trivial. In practice, however, this is often not the case. Compounding the challenges in understanding elevated convection is that some meteorologists refer to a cloud formation known as castellanus synonymously as a form of elevated convection. Two different definitions of castel- lanus exist in the literature—one is morphologically based (cloud formations that develop turreted or cumuliform shapes on their upper surfaces) and the other is physically based (inferring the turrets result from the release of conditional instability). The terms elevated convection and castellanus are not synony- mous, because castellanus can arise from surface-based convection and elevated convection exists that does not feature castellanus cloud formations. -

The Water Cycle
THE WATER CYCLE Hampton Middle School Water Cycle Vocabulary Copy in your notes • Radiation: The source of energy for evaporation is mostly solar; the water cycle is created by radiation(heat). The sun warms the earth through radiation. • Conduction: Conduction is the transfer of heat from molecule to molecule. Conduction in the water cycle takes place very close to the ground as one air molecule warms and touches another air molecule, giving off some of its heat to the other. This can be a very slow process in the atmosphere. • Convection: the mass transfer of heat from one place to another. It happens as a group of heated molecules moves to another location taking the heat with them. Convection in the water cycle is when the air near the surface is heated, then rises taking heat with it. • Hydrosphere: liquid water component of the Earth. It includes the oceans, seas, lakes, ponds, rivers and streams. The hydrosphere covers about 70% of the surface of the Earth and is the home for many plants and animals • Condensation: Condensation is the process by which water vapor in the air is changed into liquid water. Condensation is crucial to the water cycle because it is responsible for the formation of clouds. • Transpiration: process by which moisture is carried through plants from roots to small pores on the underside of leaves, where it changes to vapor and is released to the atmosphere; essentially evaporation of water from plant leaves. • Gravity: one of the driving forces in the water cycle; works mostly on groundwater • Oceanography: Oceanography, also known as oceanology and marine science, is the branch of Earth science that studies the ocean • Precipitation: any product of the condensation of atmospheric water vapor that falls under gravity; The main forms of precipitation include drizzle, rain, sleet, snow, and hail. -

Winds and Deserts Heat Moved by Convection
Winds and Deserts Wind is an agent of erosion & deposition Focus on wind as a: Flow of air Transport agent Depositional agent Agent of erosion Also discuss the desert environment Heat Moved by Convection Surfaces heated by the Sun warm the lowermost layer of the atmosphere Heated air expands in volume, becomes light (less dense), rises higher in the atmosphere, and carries heat along with it in the now familiar large scale process known as convection 1 What about Winds and Deserts? Convection transfers heat from low elevations to high elevations Creates areas of low and high pressure Air moves from high to low pressure Warm air holds more moisture than cold air Winds on Earth’’s surface carry water through atmosphere Movement of water vapor transfers lots of heat because of the latent heat of vaporization Water Vapor Content of Air Warm air is capable of holding 10 times as much water vapor as cold air Evaporation of water in warm regions stores excess heat energy in the warm atmosphere Energy stored in water vapor carried by moving air vertically and horizontally When condensation and precipitation occur, the stored latent energy released as heat far from site of evaporation 2 Heat Transfer in Earth’s Atmosphere Incoming heat is strongest near the equator while heat emitted back to space is more evenly distributed between the tropics and the poles The resulting heat surplus in the tropics and deficit at the poles creates a temperature imbalance and the equator-to-pole temperature distribution This temperature gradient -

Convective Winds
Chapter 7 CONVECTIVE WINDS Winds of local origin—convective winds caused by local temperature differences—can be as important in fire behavior as the winds produced by the synoptic-scale pressure pattern. In many areas they are the predominant winds in that they overshadow the general winds. If their interactions are understood, and their patterns known, the changes in behavior of wildfires can be predicted with reasonable accuracy. Fires occurring along a coastline will react to the changes in the land and sea breezes. Those burning in mountain valleys will be influenced by the locally produced valley and slope winds. Certainly there will be times when the convective winds will be severely altered or completely obliterated by a strong general wind flow. These cases, in which the influences of the general winds on fire behavior will predominate, must be recognized. 107 Convective Winds In the absence of strong synoptic-scale mountaintop and valley-bottom readings give pressure gradients, local circulation in the fair approximations of the temperature lapse atmosphere is often dominated by winds resulting rate and associated stability or instability. from small-scale pressure gradients produced by Height of the nighttime inversion may usually temperature differences within the locality. Air be located in mountain valleys by traversing made buoyant by warming at the surface is forced side slopes and by taking thermometer aloft; air which is cooled tends to sink. Buoyant air readings. is caused to rise by horizontal airflow resulting Strong surface heating produces the most from the temperature-induced small-scale pressure varied and complex convective wind systems. -

Buoyant Convection Computed in a Vorticity, Stream-Function Formulation
JOURNALOF RESEARCHof the National Bureau of Standards Vol. 87, No. 2, March-April 1982 Buoyant Convection Computed in a Vorticity, Stream-Function Formulation Ronald G. Rehm,* Howard R. Baum,t and P. Darcy Barnett* National Bureau of Standards, Washington, DC 20234 September2, 1981 Model equations describing large scale buoyant convection in an enclosure are formulated with the vorticity and stream function as dependent variables. The mathematical model, based on earlier work of the authors, is unique in two respects. First, it neglects viscous and thermal conductivity effects. Second the fluid is taken to be thermally expandable: large density variations are allowed while acoustic waves are filtered out. A volumetric heat source of specified spatial and temporal variation drives the flow in a two-dimensional rectangular enclosure. An algorithm for solution of the equations in this voticity, stream-function formulation is presented. Results of computations using this algorithm are presented. Comparison of these results with those obtained earlier by the authors using a finite difference code to integrate the primitive equations show excelent agreement. A method for periodically smoothing the computational results during a calculation, using Lanczos smoothing, is also presented. Computations with smoothing at different time intervals are presented and discussed. Key words: buoyant convection; finite difference computations; fire-enclosure; fluid flow; Lanczos smoothing; partial differential equations; stream function; vorticity. 1. Introduction Over the past few years, the National Bureau of Standards has sponsored a joint research project between the Center for Fire Research and the Center for Applied Mathematics to develop, starting from basic con- servation laws, a mathematical model of fire development within a room. -

Sensitivity of Moist Convection to Environmental Humidity
QUARTERLY JOURNAL OF THE ROYAL METEOROLOGICAL SOCIETY Vol. 130 OCTOBER 2004 Part C (EUROCS) No. 604 Q. J. R. Meteorol. Soc. (2004), 130, pp. 3055–3079 doi: 10.1256/qj.03.130 Sensitivity of moist convection to environmental humidity By S. H. DERBYSHIRE1∗,I.BEAU2, P. BECHTOLD3,4, J.-Y. GRANDPEIX5,J.-M.PIRIOU6, J.-L. REDELSPERGER6 andP.M.M.SOARES7,8 1Met Office, Exeter, UK 2M´et´eo-France/ENM, Toulouse, France 3Observatoire Midi-Pyr´en´ees, Toulouse, France 4European Centre for Medium-Range Weather Forecasts, Reading, UK 5Laboratoire de M´et´eorologie Dynamique, Paris, France 6M´et´eo-France/CNRM, Toulouse, France 7Instituto Superior de Engenharia de Lisboa, Portugal 8Centro de Geofisica da Universidade de Lisboa, Portugal (Received 24 July 2003; revised 27 May 2004) SUMMARY As part of the EUROCS (EUROpean Cloud Systems study) project, cloud-resolving model (CRM) simula- tions and parallel single-column model (SCM) tests of the sensitivity of moist atmospheric convection to mid- tropospheric humidity are presented. This sensitivity is broadly supported by observations and some previous model studies, but is still poorly quantified. Mixing between clouds and environment is a key mechanism, central to many of the fundamental differences between convection schemes. Here, we define an idealized quasi-steady ‘testbed’, in which the large-scale environment is assumed to adjust the local mean profiles on a timescale of one hour. We then test sensitivity to the target profiles at heights above 2km. Two independent CRMs agree reasonably well in their response to the different background profiles and both show strong deep precipitating convection in the more moist cases, but only shallow convection in the driest case.