Corporate Presentation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

REFERENCE DOCUMENT Including the Sustainable Development Report Contents
2015 REFERENCE DOCUMENT Including the Sustainable Development Report Contents Key Figures 2 1 Management Report 9 4 Financial statements 199 History of the Air Liquide Group 10 Consolidated fi nancial statements 201 Activities and risk factors 15 Statutory accounts of the parent company 275 2015 Performance 32 Investment cycle and fi nancing strategy 45 Innovation 53 Strategy and outlook 62 5 Annual General Meeting 2016 297 Board of Directors’ Report on the resolutions presented to the 2016 Combined Shareholders’ 2 2015 Corporate Social Responsibility Meeting 298 and Sustainable Development Report 65 Resolutions presented for the approval of the Combined Shareholders’ Meeting – Introduction 66 May 12, 2016 307 Our 2015 Social and Environmental Contribution 67 Statutory Auditors’ Reports 327 Environmental, social and governance (ESG) report 69 Reporting methodology 113 Independent verifi er’s report 115 Appendix 118 6 Additional information 339 Share capital 340 General information 346 3 Corporate governance 119 Trade payables 356 Factors that may have an impact in the event Management and control 120 of a takeover bid 357 Report from the Chairman of the Board of Directors 123 Person responsible for the Reference Document 359 Remuneration of the Executive Offi cers and Directors Cross-reference table for the Reference Document 360 of L’Air Liquide S.A. 147 Cross-reference table for the Annual Financial Report 364 Statutory Auditors’ Report 174 Cross-reference table for the Management Report 365 Transactions involving Company shares performed -

National Lipid Association Annual Summary of Clinical Lipidology 2016 Disclosures Dr. Harold E. Bays: Dr. Bays Discloses That He
National Lipid Association Annual Summary of Clinical Lipidology 2016 Disclosures Dr. Harold E. Bays: Dr. Bays discloses that he has received research grants from Arena Pharmaceuticals, Boehringer Ingelheim, Cargill Inc., GlaxoSmithKline, Novo Nordisk, Orexigen Therapeutics, Shionogi, Takeda, Stratum Nutrition, California Raisin Board, Esperion, Essentialis, Forest, Gilead Sciences Inc., Given, Hoffman-LaRoche, Home Access, Novartis, Omthera, Pfizer, Trygg Pharmaceuticals, TWI Bio, Xoma, Ardea Inc., High Point Pharmaceuticals LLC, Micropharma Limited, TransTech Pharma Inc., TIMI, Pozen, Regeneron, and Elcelyx. He further discloses that he has received honoraria/research grants from Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Catabasis, Daiichi-Sankyo Inc., Eisai, Merck & Co, VIVUS, Zeomedex, and WPU. Dr. Peter H. Jones: Dr. Jones has received consulting or speaker honoraria from Merck and Co., Amgen, and Sanofi-Aventis/Regeneron. Dr. W. Virgil Brown: Dr. Brown is the editor of the Journal of Clinical Lipidology and further discloses that he has received consulting fees/honoraria from Akcea, Esperion, Regeneron, Amgen, Genzyme, Pfizer Inc., Merck and Co, GlaxoSmithKline, Medtelligence, and Vindico. Dr. Terry A. Jacobson: Dr. Jacobson has received consulting fees from Merck and Co., Amarin, Amgen, AstraZeneca, and Regeneron/Sanofi-Aventis. Dr. Karen E. Aspry: Dr. Aspry has no disclosures to report. Dr. Christie M. Ballantyne: Dr. Ballantyne has received research grants from Abbott Diagnostics, Amarin, Amgen, Eli Lilly, Esperion, ISIS Pharmaceuticals, Novartis Pharmaceuticals, Pfizer, Otsuka, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, and Takeda Pharmaceuticals. He has also received consulting fees from Abbott Diagnostics, Amarin, Amgen, AstraZeneca, Eli Lilly, Esperion, Genzyme, ISIS Pharmaceuticals, Matinas BioPharma Inc., Merck and Co., Novartis Pharmaceuticals, Pfizer, Regeneron, Roche, and Sanofi-Synthelabo. -

Euronext Single Stock Dividend Futures: the Widest Choice of Contracts
DIVIDEND DERIVATIVES: PLAY THE DIVIDEND FIELD WITH EURONEXT EURONEXT SINGLE STOCK DIVIDEND FUTURES: THE WIDEST CHOICE OF CONTRACTS September 2019 EURONEXT SSDFS: THE WIDEST CHOICE OF CONTRACTS 1st Dividend Exchange by number of contracts Number of contracts per country of underlying Number Number of SSDFs Underlyings ▪ Euronext began developing the range in of SSDFs only on Euronext Italy 24 11 January 2015 and regularly issues contracts Germany 27 0 to satisfy the needs of end-users Netherlands 23 9 ▪ We have nearly 300 SSDF contracts tradable Belgium 15 14 with the most diversified underlyings from Portugal 3 3 France 47 7 across Europe and the USA. Ireland 1 0 ▪ Investors can access dividend contracts on UK 36 10 new underlyings, as a total of 117 SSDFs are Spain 22 10 only available at Euronext exclusively. USA 57 36 Finland 7 2 Sweden 13 12 Norway 2 2 Switzerland 18 0 Austria 1 1 │ 2 INTRODUCTION OF SEMI-ANNUAL MATURITIES Euronext introduced semi-annual maturities on a range of Single Stock Dividend Futures listed on the Paris Derivatives Market, which offer new trading opportunities to market participants by helping them target dividend distributions more accurately on most traded names (see below). For these underlyings, the maturities available are 6, 12, 18, 24, 30, 36, 42, 48, 54 and 60 months. UNDERLYING ISIN CODE TRADING SYMBOL 1 Sanofi FR0000120578 SA8 2 BNP Paribas FR0000131104 BN8 3 AXA FR0000120628 CS8 4 Air Liquide FR0000120073 AI8 5 Orange SA FR0000133308 FT8 6 Vinci SA FR0000133308 DG8 7 LVMH FR0000121014 MC8 8 ENGIE -

Executive Profile: Genzyme's CEO on Dark Times, Transition and the Long
June 15th 2015 scripintelligence.com Executive profile: Genzyme’s CEO on dark times, transition and the long view David Meeker, CEO of Sanofi company Genzyme, recently found himself hustling back to Genzyme’s Kendall Square offices from a nearby meeting to talk to Scrip’s editor Eleanor Malone about the challenges and opportunities he has encountered in his varied career. In the first of a two-part series, he discusses his experiences in the company that he joined in 1994, and expounds upon issues faced by the industry as a whole. Eleanor Malone: Genzyme went through some dark times before the Sanofi acquisition with the manufacturing issues that led to shortages of enzyme replacement therapies. What lessons did you draw from those difficulties? David Meeker: You are absolutely correct; they were incredibly painful, dark moments. We failed to deliver on the implicit promise David Meeker addresses employees at of ensuring that patients can access the Genzyme’s HQ following a torch relay connecting the company’s sites near Boston medicines that they need. I think we learned to mark International Rare Diseases Day many lessons. On a very tactical level we learned about the lead time that it takes to bring biologic manufacturing up – we realised it right for the patient, given the limitations Many acquisitions destroy value, and a lot too late that we were not going to be at an we had. We were short of product so we had of the destruction happens when people adequate capacity and by the time we started to focus on how to manage the distribution leave: you have the products but you lose to build, even though it was several years of that product in a way which was most the know-how and the critical talent that ahead of when we ran out of medicine, we equitable. -

Letter to Shareholders April 2021
LETTER TO SHAREHOLDERS APRIL 2021 P. 2 — RESULTS P. 4 — COVID-19 P. 6 — CMD21 P. 8 — SHAREHOLDER INFORMATION RESULTS MESSAGE FROM FULL-YEAR 2020 THE CHAIRMAN RESULTS Business net Company sales1 income1 €36,041m €7,347m +3.3% (-0.2%) +9.6% (+4.2%) Serge Weinberg, Chairman of the Board of Directors Business EPS 1 , 2 2020 dividend3 Dear shareholders, Summarizing 2020 in a few words is difficult. The progress Sanofi has €5,86 €3.20 made in this first year of execution of the new strategy presented in +9.2% (+3.9%) per share December 2019 is very real. Our Research and Development pipeline is improving and the global business units are refocusing on their core activities. More than half of the cost savings achieved due to our collective efforts to be smarter in how we spend, has been reinvested in science. We strengthened our positioning in therapeutic areas through several acquisitions by adding pipeline projects where we already have a promising future. This would not have been possible without the support of all employees to the ‘Play to Win’ strategy. INTERVIEW WITH THE CHIEF Since the start of the health crisis, all our industrial sites have remained operational to maintain the continuous production of essential EXECUTIVE OFFICER medicines for patients. We have initiated two vaccine projects against COVID-19: for the most advanced of them1, the objective is to obtain approval of the health authorities in the fourth quarter of 2021 and first doses of the vaccine available for people worldwide to be ready. In the meantime, we are committed to helping some of our competitors whose vaccines have already been approved by participating in the manufacturing of millions of additional doses. -

Dyax Corp. 300 Technology Square Cambridge, MA 02139 617 225-2500
Dyax Corp. 300 Technology Square Cambridge, MA 02139 617 225-2500 www.dyax.com Other Offices Dyax SA, Liege, Belgium ADVANCING NOVEL THERAPEUTIC PRODUCTS Dyax Corp. Annual Report 2003 Corporate Information Dyax Achievements 2003 Dyax Goals 2004 Directors Executive Officers and Stock Listing Henry E. Blair Key Employees Common stock has been traded on the Nasdaq Stock Market Advances in Clinical Development Clinical Development Chairman, President and Henry E. Blair* under the symbol DYAX since our initial public offering in Milestones Chief Executive Officer, Chairman, President and August 14, 2000. DX-88/Hereditary Angioedema Dyax Corp. Chief Executive Officer DX-88/Hereditary Constantine E. Stephen S. Galliker, CPA* The following table gives the quarterly high and low sales G Completed 9-patient Phase II trial, met primary endpoints Angioedema Anagnostopoulos, Ph.D. EVP Finance and prices of our common stock for the last three years. G Genzyme Corporation joined Dyax as joint venture partner Managing General Partner, Administration and G Complete Phase II 2001 2002 2003 G Gateway Associates, LP Chief Financial Officer Completed 3 of 4 dose cohorts in 48-patient Phase II EDEMA1 trial EDEMA1 study High Low High Low High Low G Susan B. Bayh, J.D. Lynn G. Baird, Ph.D.* Initiated Phase II EDEMA2 trial G Periodically observe James W. Fordyce SVP Development First Quarter $20.94 $6.56 $11.38 $3.10 $2.25 $1.52 G Orphan Drug designation granted in U.S. and Europe effects of repeat dosing in Phase II EDEMA2 Managing Partner, Robert C. Ladner, Ph.D. Second Quarter $19.99 $6.81 $ 4.68 $3.20 $4.90 $1.67 Fordyce & Gabrielson, LLC SVP and Chief DX-88/On-Pump Open Heart Surgery (CABG) study Third Quarter $21.24 $6.05 $ 4.20 $1.65 $7.50 $2.58 Mary Ann Gray, Ph.D. -

Case No COMP/M.6212 - LVMH/ BULGARI
EN Case No COMP/M.6212 - LVMH/ BULGARI Only the English text is available and authentic. REGULATION (EC) No 139/2004 MERGER PROCEDURE Article 6(1)(b) NON-OPPOSITION Date: 29/06/2011 In electronic form on the EUR-Lex website under document number 32011M6212 Office for Publications of the European Union L-2985 Luxembourg EUROPEAN COMMISSION Brussels, 29.6.2011 In the published version of this decision, some information has been omitted pursuant to Article C(2011) 4823 final 17(2) of Council Regulation (EC) No 139/2004 concerning non-disclosure of business secrets and other confidential information. The omissions are PUBLIC VERSION shown thus […]. Where possible the information omitted has been replaced by ranges of figures or a general description. MERGER PROCEDURE ARTICLE 6(1)(b) DECISION To the notifying party: Dear Sir/Madam, Subject: Case No COMP/M.6212 - LVMH/ BULGARI Commission decision pursuant to Article 6(1)(b) of Council Regulation No 139/20041 1. On 24 May 2011, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which LVMH Moët Hennessy – Louis Vuitton Group ("LVMH", France), controlled by Groupe Arnault SAS (France), acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of the undertaking Bulgari S.p.A ("Bulgari", Italy) by way of purchase of shares.2 LVMH and Bulgari will be hereinafter referred to as "the parties". I. THE PARTIES 2. LVMH is active in the production and sales of luxury goods (wines and spirits; fashion and leather goods, including accessories; perfumes and cosmetics; watches and jewellery; selective retailing as well as the luxury yachts industry). -

Translate Bio, Inc. (Name of Issuer)
SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 SCHEDULE 13D (Rule 13d-101) UNDER THE SECURITIES EXCHANGE ACT OF 1934 Translate Bio, Inc. (Name of Issuer) Common Stock, $0.001 par value per share (Title of Class of Securities) 89374L104 (CUSIP Number) Karen Linehan Executive Vice President, Legal Affairs and General Counsel Sanofi 54, rue La Boétie, 75008 Paris, France Telephone: +33 1 53 77 40 00 Copy to: Michael J. Aiello, Esq. Matthew J. Gilroy, Esq. Weil, Gotshal & Manges LLP 767 Fifth Avenue New York, New York 10153 (212) 310-8000 (Name, Address and Telephone Number of Person Authorized to Receive Notices and Communications) August 2, 2021 (Date of Event Which Requires Filing of This Statement) If the filing person has previously filed a statement on Schedule 13G to report the acquisition which is the subject of this Schedule 13D, and is filing this schedule because of Rule 13d-1(e), 13d-1(f) or 13d-1(g), check the following box. ☐ Note. Schedules filed in paper format shall include a signed original and five copies of the schedule, including all exhibits. See Rule 13d-7 for other parties to whom copies are to be sent. *The remainder of this cover page shall be filled out for a reporting person’s initial filing on this form with respect to the subject class of securities, and for any subsequent amendment containing information which would alter disclosures provided in a prior cover page. The information required on the remainder of this cover page shall not be deemed to be “filed” for the purpose of Section 18 of the Securities Exchange Act of 1934 (“Act”) or otherwise subject to the liabilities of that section of the Act but shall be subject to all other provisions of the Act (however, see the Notes). -

Cerezyme, INN-Imiglucerase
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cerezyme 400 Units Powder for concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 400 units* of imiglucerase**. After reconstitution, the solution contains 40 units (approximately 1.0 mg) of imiglucerase per ml (400 U/10 ml). * An enzyme unit (U) is defined as the amount of enzyme that catalyses the hydrolysis of one micromole of the synthetic substrate para-nitrophenyl β-D-glucopyranoside (pNP-Glc) per minute at 37°C. ** Imiglucerase is a modified form of human acid β-glucosidase and is produced by recombinant DNA technology using a mammalian Chinese Hamster Ovary (CHO) cell culture, with mannose modification for targeting macrophages. Excipients: For the full list of excipients, see section 6.1. This medicinal product contains sodium and is administered in 0.9% sodium chloride intravenous solution (see section 6.6). After reconstitution, the solution contains 1.24 mmol sodium (400 U/10 mL). To be taken into consideration by patients on a controlled sodium diet. 3. PHARMACEUTICAL FORM Powder for concentrate for solution for infusion. Cerezyme is a white to off-white powder. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Cerezyme (imiglucerase) is indicated for use as long-term enzyme replacement therapy in patients with a confirmed diagnosis of non-neuronopathic (Type 1) or chronic neuronopathic (Type 3) Gaucher disease who exhibit clinically significant non-neurological manifestations of the disease. The non-neurological manifestations of Gaucher disease include one or more of the following conditions: • anaemia after exclusion of other causes, such as iron deficiency • thrombocytopenia • bone disease after exclusion of other causes such as Vitamin D deficiency • hepatomegaly or splenomegaly 4.2 Posology and method of administration Disease management should be directed by physicians knowledgeable in the treatment of Gaucher disease. -

EURO STOXX 50 Index
EURO STOXX 50® Europe’s Leading Blue-Chip Index June 2020 1 | Confidential – Not for Redistribution – Copyright © 2020 Qontigo GmbH. Qontigo is part of Deutsche Börse Group. Content 02 Introduction Drivers of Strategic and Tactical 12 Allocations to the Eurozone 17 Appendix STOXX is now Part of Qontigo… A new financial intelligence driver, modernizing investment management Index Analytics STOXX & DAX AXIOMA World-class indices that are licensed to more Best of breed portfolio construction and risk than 500 companies, including the world’s analytics tools. largest financial product issuers, capital owners and asset managers. 3 | Confidential – Not for Redistribution – Copyright © 2020 Qontigo GmbH. Qontigo is part of Deutsche Börse Group. EURO STOXX 50® - A Unique Offering for Liquid, Diversified Access to the Eurozone Features Regional Coverage1) ▪ Comprehensive and transparent: completely rules-based coverage of the Eurozone1) ▪ Balanced: selection mechanism ensures balanced representation of supersectors using Industry Classification Benchmark (ICB) ▪ Representative and liquid: coverage of about 60% of total free-float through liquid supersector leaders. Components ranked and weighted by free-float subject to 10% cap ▪ Continuous pulse on market changes: quarterly rebalancing, annual review ▪ Established: launched on Feb. 26, 1998 1) Country composition as of March 2020: Belgium, Finland, France, Germany, Ireland, Italy, Luxembourg, the Netherlands and Spain 4 | Confidential – Not for Redistribution – Copyright © 2020 Qontigo GmbH. Qontigo is part of Deutsche Börse Group. Methodology Leads to Balanced Representation of Eurozone Supersector Leaders Sub- Universe universes Pre-selection Selection Weighting ... rank stocks by free- EURO STOXX® Select 50 largest float and select largest (covers approximately Within each of the 19 stocks as measured Weight by free-float stocks jointly by free-float from all subject to 10% cap 95% of Eurozone free- supersectors .. -
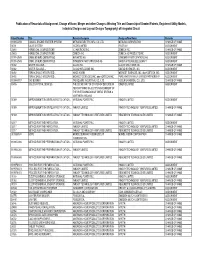
Publication of Recordals of Assignment, Change of Name
Publication of Recordals of Assignment, Change of Name, Merger and other Changes Affecting Title and Ownership of Granted Patents, Registered Utility Models, Industrial Designs and Lay-out Designs Topography) of Integrated Circuit Patent Number Title Patentee/Assignor Assignee/New Name Remarks 1199654059 COAXIAL ENGINE STARTER SYSTEM MITSUBA ELECTRIC MFG. CO. LTD. MITSUBA CORPORATION CHANGE OF NAME 31731 VALVE SYSTEM ISCOR LIMITED IPCOR NV ASSIGNMENT 25483 HERBICIDAL COMPOSITIONS ICI AMERICAS INC. ZENECA INC. CHANGE OF NAME 25483 HERBICIDAL COMPOSITIONS ZENECA INC. ZENECA AG PRODUCTS INC. ASSIGNMENT 1199142549 OXIME ETHERS DERIVATIVES NOVARTIS AG SYNGENTA PARTICIPATIONS AG ASSIGNMENT 1199142549 OXIME ETHERS DERIVATIVES SYNGENTA PARTICIPATIONS AG BAYER AKTIENGESELLSCHAFT ASSIGNMENT 31882 WATER SOLUBLE… GLAXO INC. GLAXO WELLCOME INC. CHANGE OF NAME 31882 WATER SOLUBLE… GLAXO WELLCOME INC. GILEAD SCIENCES, INC. ASSIGNMENT 31492 REPLACEABLE INTEGRATED… AMOS KORIN MIDWEST SCIENCES, INC. dba KORTECH, INC. ASSIGNMENT 31492 REPLACEABLE INTEGRATED… MIDWEST SCIENCES, INC. dba KORTECH, INC. PURE WATER FAMILY LIMITED PARTNERSHIP ASSIGNMENT 1199447817 GAS BURNER TRI-SQUARE INDUSTRIAL CO., LTD. HOSUN UNIVERSAL CO., LTD. CHANGE OF NAME 18495 LIQUID CRYSTAL DEVICES THE SECRETARY OF STATE FOR DEFENSE IN QINETIQ LIMITED ASSIGNMENT HER BRITTANIC MAJESTY'S GOVERNMENT OF THE UNITED KINGDOM OF GREAT BRITAIN & NORTHERN IRELAND 31389 IMPROVEMENTS IN OR RELATING TO CATION… NATIONAL POWER PLC INNOGY LIMITED ASSIGNMENT 31389 IMPROVEMENTS IN OR RELATING TO CATION… -

Transforming Pharma to Deliver Sustainable Long-Term Growth /// March 10-11, 2021 Cautionary Statements Regarding Forward-Looking Information
Transforming Pharma to Deliver Sustainable Long- term Growth /////////// Capital Markets Day March 10-11, 2021 Stefan Oelrich President of the Pharmaceuticals Division Marianne De Backer Head of Strategy and BD&L Pharmaceuticals Christian Rommel Head of R&D Pharmaceuticals 1 /// Bay er Capital Markets Day /// Transforming Pharma to Deliver Sustainable Long-term Growth /// March 10-11, 2021 Cautionary Statements Regarding Forward-Looking Information This presentation may contain forward-looking statements based on current assumptions and forecasts made by Bayer management. Various known and unknown risks, uncertainties and other factors could lead to material differences between the actual future results, financial situation, development or performance of the company and the estimates given here. These factors include those discussed in Bayer’s public reports which are available on the Bayer website at http://www.bayer.com/. The company assumes no liability whatsoever to update these forward-looking statements or to conformthem to future events or developments. 2 /// Bay er Capital Markets Day /// Transforming Pharma to Deliver Sustainable Long-term Growth /// March 10-11, 2021 1 Overview Pharma: Focused on Therapeutic Areas with High Unmet Needs Sales development EBITDA margin development Sales by region In €m In %, before special items In %, in 2020 17,962 16,420 16,847 16,746 17,243 North 34.9 America 22 33.9 33.4 32.0 32.6 40 EMEA Latin 5 America 33 2016 2017 2018 2019 2020 2016 2017 2018 2019 2020 Asia / Pacific Therapeutic areas