Iv. Biology of Crepidula Fornicata Y of Crepidula
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crepidula Fornicata
NOBANIS - Marine invasive species in Nordic waters - Fact Sheet Crepidula fornicata Author of this species fact sheet: Kathe R. Jensen, Zoological Museum, Natural History Museum of Denmark, Universiteteparken 15, 2100 København Ø, Denmark. Phone: +45 353-21083, E-mail: [email protected] Bibliographical reference – how to cite this fact sheet: Jensen, Kathe R. (2010): NOBANIS – Invasive Alien Species Fact Sheet – Crepidula fornicata – From: Identification key to marine invasive species in Nordic waters – NOBANIS www.nobanis.org, Date of access x/x/201x. Species description Species name Crepidula fornicata, Linnaeus, 1758 – Slipper limpet Synonyms Patella fornicata Linnaeus, 1758; Crepidula densata Conrad, 1871; Crepidula virginica Conrad, 1871; Crepidula maculata Rigacci, 1866; Crepidula mexicana Rigacci, 1866; Crepidula violacea Rigacci, 1866; Crepidula roseae Petuch, 1991; Crypta nautarum Mörch, 1877; Crepidula nautiloides auct. NON Lesson, 1834 (ISSG Database; Minchin, 2008). Common names Tøffelsnegl (DK, NO), Slipper limpet, American slipper limpet, Common slipper snail (UK, USA), Østerspest (NO), Ostronpest (SE), Amerikanische Pantoffelschnecke (DE), La crépidule (FR), Muiltje (NL), Seba (E). Identification Crepidula fornicata usually sit in stacks on a hard substrate, e.g., a shell of other molluscs, boulders or rocky outcroppings. Up to 13 animals have been reported in one stack, but usually 4-6 animals are seen in a stack. Individual shells are up to about 60 mm in length, cap-shaped, distinctly longer than wide. The “spire” is almost invisible, slightly skewed to the right posterior end of the shell. Shell height is highly variable. Inside the body whorl is a characteristic calcareous shell-plate (septum) behind which the visceral mass is protected. -

Benthic Invertebrate Community Monitoring and Indicator Development for Barnegat Bay-Little Egg Harbor Estuary
July 15, 2013 Final Report Project SR12-002: Benthic Invertebrate Community Monitoring and Indicator Development for Barnegat Bay-Little Egg Harbor Estuary Gary L. Taghon, Rutgers University, Project Manager [email protected] Judith P. Grassle, Rutgers University, Co-Manager [email protected] Charlotte M. Fuller, Rutgers University, Co-Manager [email protected] Rosemarie F. Petrecca, Rutgers University, Co-Manager and Quality Assurance Officer [email protected] Patricia Ramey, Senckenberg Research Institute and Natural History Museum, Frankfurt Germany, Co-Manager [email protected] Thomas Belton, NJDEP Project Manager and NJDEP Research Coordinator [email protected] Marc Ferko, NJDEP Quality Assurance Officer [email protected] Bob Schuster, NJDEP Bureau of Marine Water Monitoring [email protected] Introduction The Barnegat Bay ecosystem is potentially under stress from human impacts, which have increased over the past several decades. Benthic macroinvertebrates are commonly included in studies to monitor the effects of human and natural stresses on marine and estuarine ecosystems. There are several reasons for this. Macroinvertebrates (here defined as animals retained on a 0.5-mm mesh sieve) are abundant in most coastal and estuarine sediments, typically on the order of 103 to 104 per meter squared. Benthic communities are typically composed of many taxa from different phyla, and quantitative measures of community diversity (e.g., Rosenberg et al. 2004) and the relative abundance of animals with different feeding behaviors (e.g., Weisberg et al. 1997, Pelletier et al. 2010), can be used to evaluate ecosystem health. Because most benthic invertebrates are sedentary as adults, they function as integrators, over periods of months to years, of the properties of their environment. -

The American Slipper Limpet Crepidula Fornicata (L.) in the Northern Wadden Sea 70 Years After Its Introduction
Helgol Mar Res (2003) 57:27–33 DOI 10.1007/s10152-002-0119-x ORIGINAL ARTICLE D. W. Thieltges · M. Strasser · K. Reise The American slipper limpet Crepidula fornicata (L.) in the northern Wadden Sea 70 years after its introduction Received: 14 December 2001 / Accepted: 15 August 2001 / Published online: 25 September 2002 © Springer-Verlag and AWI 2002 Abstract In 1934 the American slipper limpet 1997). In the centre of its European distributional range Crepidula fornicata (L.) was first recorded in the north- a population explosion has been observed on the Atlantic ern Wadden Sea in the Sylt-Rømø basin, presumably im- coast of France, southern England and the southern ported with Dutch oysters in the preceding years. The Netherlands. This is well documented (reviewed by present account is the first investigation of the Crepidula Blanchard 1997) and sparked a variety of studies on the population since its early spread on the former oyster ecological and economic impacts of Crepidula. The eco- beds was studied in 1948. A field survey in 2000 re- logical impacts of Crepidula are manifold, and include vealed the greatest abundance of Crepidula in the inter- the following: tidal/subtidal transition zone on mussel (Mytilus edulis) (1) Accumulation of pseudofaeces and of fine sediment beds. Here, average abundance and biomass was 141 m–2 through the filtration activity of Crepidula and indi- and 30 g organic dry weight per square metre, respec- viduals protruding in stacks into the water column. tively. On tidal flats with regular and extended periods of This was reported to cause changes in sediments and emersion as well as in the subtidal with swift currents in near-bottom currents (Ehrhold et al. -

Marine Ecology Progress Series 464:135
Vol. 464: 135–151, 2012 MARINE ECOLOGY PROGRESS SERIES Published September 19 doi: 10.3354/meps09872 Mar Ecol Prog Ser Physical and biological factors affect the vertical distribution of larvae of benthic gastropods in a shallow embayment Michelle J. Lloyd1,*, Anna Metaxas1, Brad deYoung2 1Department of Oceanography, Dalhousie University, Halifax, Nova Scotia, Canada B3H 4R2 2Department of Physics and Physical Oceanography, Memorial University, St. John’s, Newfoundland, Canada A1B 3X7 ABSTRACT: Marine gastropods form a diverse taxonomic group, yet little is known about the factors that affect their larval distribution and abundance. We investigated the larval vertical dis- tribution and abundance of 9 meroplanktonic gastropod taxa (Margarites spp., Crepidula spp., Astyris lunata, Diaphana minuta, Littorinimorpha, Arrhoges occidentalis, Ilyanassa spp., Bittiolum alternatum and Nudibranchia), with similar morphology and swimming abilities, but different adult habitats and life-history strategies. We explored the role of physical (temperature, salinity, density, current velocities) and biological (fluorescence) factors, as well as periodic cycles (lunar phase, tidal state, diel period) in regulating larval vertical distribution. Using a pump, we collected plankton samples at 6 depths (3, 6, 9, 12, 18 and 24 m) at each tidal state, every 2 h over a 36 and a 26 h period, during a spring and neap tide, respectively, in St. George’s Bay, Nova Scotia. Con- currently, we measured temperature, salinity, density, fluorescence (as a proxy for chlorophyll, i.e. phytoplankton density), and current velocity. Larval abundance was most strongly related to tem- perature, except for Littorinimorpha and Crepidula spp., for which it was most strongly related to fluorescence. Margarites spp., A. -

1 Invasive Species in the Northeastern and Southwestern Atlantic
This is the author's accepted manuscript. The final published version of this work (the version of record) is published by Elsevier in Marine Pollution Bulletin. Corrected proofs were made available online on the 24 January 2017 at: http://dx.doi.org/10.1016/j.marpolbul.2016.12.048. This work is made available online in accordance with the publisher's policies. Please refer to any applicable terms of use of the publisher. Invasive species in the Northeastern and Southwestern Atlantic Ocean: a review Maria Cecilia T. de Castroa,b, Timothy W. Filemanc and Jason M Hall-Spencerd,e a School of Marine Science and Engineering, University of Plymouth & Plymouth Marine Laboratory, Prospect Place, Plymouth, PL1 3DH, UK. [email protected]. +44(0)1752 633 100. b Directorate of Ports and Coasts, Navy of Brazil. Rua Te filo Otoni, 4 - Centro, 20090-070. Rio de Janeiro / RJ, Brazil. c PML Applications Ltd, Prospect Place, Plymouth, PL1 3DH, UK. d Marine Biology and Ecology Research Centre, Plymouth University, PL4 8AA, UK. e Shimoda Marine Research Centre, University of Tsukuba, Japan. Abstract The spread of non-native species has been a subject of increasing concern since the 1980s when human- as a major vector for species transportation and spread, although records of non-native species go back as far as 16th Century. Ever increasing world trade and the resulting rise in shipping have highlighted the issue, demanding a response from the international community to the threat of non-native marine species. In the present study, we searched for available literature and databases on shipping and invasive species in the North-eastern (NE) and South-western (SW) Atlantic Ocean and assess the risk represented by the shipping trade between these two regions. -

Download Preprint
1 Mobilising molluscan models and genomes in biology 2 Angus Davison1 and Maurine Neiman2 3 1. School of Life Sciences, University Park, University of Nottingham, NG7 2RD, UK 4 2. Department of Biology, University of Iowa, Iowa City, IA, USA and Department of Gender, 5 Women's, and Sexuality Studies, University of Iowa, Iowa, City, IA, USA 6 Abstract 7 Molluscs are amongst the most ancient, diverse, and important of all animal taxa. Even so, 8 no individual mollusc species has emerged as a broadly applied model system in biology. 9 We here make the case that both perceptual and methodological barriers have played a role 10 in the relative neglect of molluscs as research organisms. We then summarize the current 11 application and potential of molluscs and their genomes to address important questions in 12 animal biology, and the state of the field when it comes to the availability of resources such 13 as genome assemblies, cell lines, and other key elements necessary to mobilising the 14 development of molluscan model systems. We conclude by contending that a cohesive 15 research community that works together to elevate multiple molluscan systems to ‘model’ 16 status will create new opportunities in addressing basic and applied biological problems, 17 including general features of animal evolution. 18 Introduction 19 Molluscs are globally important as sources of food, calcium and pearls, and as vectors of 20 human disease. From an evolutionary perspective, molluscs are notable for their remarkable 21 diversity: originating over 500 million years ago, there are over 70,000 extant mollusc 22 species [1], with molluscs present in virtually every ecosystem. -

Fecundity of the Invasive Marine Gastropod Crepidula Fornicata Near the Current Northern Extreme of Its Range
Invertebrate Biology 136(4): 394–402. © 2017, The American Microscopical Society, Inc. DOI: 10.1111/ivb.12194 Fecundity of the invasive marine gastropod Crepidula fornicata near the current northern extreme of its range Jan A. Pechenik,1,a Casey M. Diederich,1 Howard I. Browman,2 and Anders Jelmert3 1 Department of Biology, Tufts University, Medford, Massachusetts 02155, USA 2 Institute of Marine Research, Austevoll Research Station, Storebø, Norway 3 Institute of Marine Research, 5817 Bergen, Norway Abstract. The calyptraeid gastropod Crepidula fornicata is native to the eastern coast of the United States but has now become an extremely successful invader along much of the Euro- pean coastline. As the northern limit of its spread is thought to be determined by an inabil- ity of adults to tolerate prolonged exposure to low winter temperatures, this study sought to compare the fecundity of females collected from two sites along the Norwegian coastline with that of females collected from Rhode Island, USA. Few other studies have compared the fecundities of marine invertebrates from invasive populations with those found in native populations. For both populations studied, fecundities increased with increasing shell length. However, contrary to expectations, size-related fecundities were significantly higher for Norwegian females than for Rhode Island females, with Norwegian females producing larger egg capsules and a greater number of embryos per capsule, but not a greater number of egg capsules per brood. Current evidence suggests that at -

Assessing the Impact of Key Marine Invasive Non-Native Species on Welsh MPA Habitat Features, Fisheries and Aquaculture
Assessing the impact of key Marine Invasive Non-Native Species on Welsh MPA habitat features, fisheries and aquaculture. Tillin, H.M., Kessel, C., Sewell, J., Wood, C.A. Bishop, J.D.D Marine Biological Association of the UK Report No. 454 Date www.naturalresourceswales.gov.uk About Natural Resources Wales Natural Resources Wales’ purpose is to pursue sustainable management of natural resources. This means looking after air, land, water, wildlife, plants and soil to improve Wales’ well-being, and provide a better future for everyone. Evidence at Natural Resources Wales Natural Resources Wales is an evidence based organisation. We seek to ensure that our strategy, decisions, operations and advice to Welsh Government and others are underpinned by sound and quality-assured evidence. We recognise that it is critically important to have a good understanding of our changing environment. We will realise this vision by: Maintaining and developing the technical specialist skills of our staff; Securing our data and information; Having a well resourced proactive programme of evidence work; Continuing to review and add to our evidence to ensure it is fit for the challenges facing us; and Communicating our evidence in an open and transparent way. This Evidence Report series serves as a record of work carried out or commissioned by Natural Resources Wales. It also helps us to share and promote use of our evidence by others and develop future collaborations. However, the views and recommendations presented in this report are not necessarily those of -

Crepidula Formicatacrepidula Formicata
Crepidula formicataCrepidula formicata Easily seen Crepidula fornicata along the shoreline. Class: Gastropoda Order: Littorinimorpha Family: Calyptraeidae Genus: Crepidula Distribution It is native to the eastern This species has been introduced to several other countries, coast of North America. especially in Europe. It is considered to be an invasive, nuisance It occurs from the Gulf of species. The first known occurrence of Crepidula fornicata in St. Lawrence in the north Europe was in 1872. It was introduced accidently with imported all the way south to the American oysters. They have been repeatedly introduced ever Gulf of Mexico. since in ballast water and on ships’ hulls. Habitat They are most abundant in muddy or mixed muddy areas, This species occupies a reaching their highest densities in wave protected locations. variety of seabed surfaces. These include bays, estuaries and sheltered sides of wave- They also occur in a wide exposed islands. In Nova Scotia they are common in intertidal range of environmental and shallow subtidal regions. They attach themselves to almost conditions. any hard surface to be found (including each other and the shells of other living or dead hard-shelled invertebrates. Food They are active suspension feeders, generating a water current They live off of plankton through the mantle cavity by ciliary (hair-like) action and which they filter out of the trapping food particles on a mucous sheet lying across the front seawater. surface of the gill filament. Reproduction Being hermaphrodites they contain both male and female The Slipper limpet is a. reproductive organs. Sperm producing ability advances quicker protandric hermaphrodite. -

Table of Contents MOLLUSCA ( GASTROPODA ) Crepidula
MOLLUSCA http://www.mbl.edu/BiologicalBulletin/EGGCOMP/pages/61.html Table of Contents MOLLUSCA ( GASTROPODA ) Crepidula fornicata and C. plana The greenish-brown, boat-shaped C. fornicata adults pile one on top of another to form "chains" of individuals; the colony is attached to a stone, shell or other solid object by the bottom limpet. This species can be collected at Vineyard Haven Harbor, Mass. Another species common to the Woods Hole area, C. plana, is found within whelk or moon snail shells inhabited by large hermit crabs, and can be obtained at Cotuit. This species has a flat, whitish shell and is considerably smaller than C. fornicata. Both species are potentially protandric hermaphrodites. The active males are small, whereas the mature females are the large, older individuals; all sizes and sexual conditions can be found in any colony. C. fornicata breeds from mid-June until mid-August. C. plana has a longer season; it breeds through the first week in September (Bumpus, 1898). Sexual activity is reduced to a minimum at sea water temperatures below 15 to 16û C. (Gould, 1950). A. Care of Adults: These limpets may be kept indefinitely in aquaria or fingerbowls provided with a current of flowing, unfiltered sea water. C. plana will readily attach itself to glass when it is removed from the hermit crab shell. Unless they are already in the adult female phase, these animals eventually differentiate into males and females. B. Procuring Fertilized Ova: The animals can be detached with a heavy knife. If eggs have been deposited, they are found in a transparent capsule, attached to the substrate or to the foot of the female. -

A Comparative Study of Some Population Characteristics Of
Brigham Young University BYU ScholarsArchive Theses and Dissertations 1975-04-01 A comparative study of some population characteristics of Calyptraea fastigiata Gould, Crepidula lingulata Gould, and Crepidula nummaria Gould (Gastropoda, Prosobranchia) Roger Harold Goodwill Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd BYU ScholarsArchive Citation Goodwill, Roger Harold, "A comparative study of some population characteristics of Calyptraea fastigiata Gould, Crepidula lingulata Gould, and Crepidula nummaria Gould (Gastropoda, Prosobranchia)" (1975). Theses and Dissertations. 7935. https://scholarsarchive.byu.edu/etd/7935 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. A COMPARATIVE STUDY OF SOME POPULATION CHARACTERISTICS OF CALYPTRAEA FASTIGIATA GOULD, CREPIDULA LINGULATA GOULD, Pi.ND CREPIDULA NUMM.�IA GOULD (GASTROPODA, PROSOBRANCHIA) A Thesis Presented to the Department of Zoology Brigham Young University In Partial Fulfillment of the Requirements for the Degree Master of Science v by Roger Harold Goodwill April 1975 This thesis, by Roger Harold Goodwill, is accepted in its present form by the Department of Zoology of Brigham Young University as satisfying the thesis requirement for the degree of Master of Science. Typed by Katherine Shepherd ii ACKNOWLEDGMENTS I would like to express my appreciation to Dr. Lee Braithwaite for his help and friendship throughout my grad uate studies at BYU. I also offer my thanks to Dr. James Barnes and Dr. Melvin Carter who gave of their time freely and offered many helpful suggestions. -
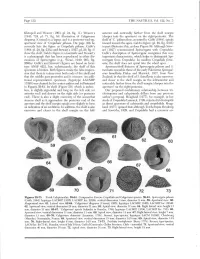
(1943: 724, Pi. 71, Fig. 16) Illustration of Calyptraea (Deeper Into the Aperture) on the Right/Posterior
Page 118 THE NAUTILUS, Vol. 122, No. 3 Kleinpeil and Weaver (1963, pi. 24, fig. 11). Weavers anterior and noticeably farther from the shell margin (1943: 724, pi. 71, fig. 16) illustration of Calyptraea (deeper into the aperture) on the right/posterior. The diegoana (Conrad) is a lapsus and is a posterior-end-up, shelf of 'C.y pileum thus, as noted by Gabb (1864), spirals apertural view of 'Crepidula' pileum. On page 356 he inward toward the apex. Gabb's figure (pi. 29, fig. 233b) correctly lists the figure as Crepidula pileum. Gabb's in part illustrates this, as does Figure 59. Although Stew- (1864: pi. 29, fig. 233a) and Stewart's (1927, pi. 29, fig. 3) art (1927) synonomized Spirocrypta with Crepidula, show the shelf. Gabb's figure is a fascimile and Stewart's Gabb's description of Spirocrypta recognizes this very is a photograph that has been reproduced in other dis- important characteristic, which helps to distinguish Spi- cussions of Spirocrypta (e.g., Wenz, 1940: 903, fig. rocrypta from Crepidula. In modern Crepidula forni- 2660a). Gabb's and Stewart's figures are based on lecto- cata, the shelf does not spiral into the whorl apex. type ANSP 4221, but, unfortunately, the shelf of this Aperture/shelf features of Spirocrypta pileum and S. specimen is broken. Both figures create the false impres- inomata resemble those of the early Paleocene Spirogal- sion that there is a sinus near both ends of the shelf and erus lamellaria Finlay and Marwick, 1937, from New that the middle part protrudes and is concave.