A Comparative Study of Some Population Characteristics Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crepidula Fornicata
NOBANIS - Marine invasive species in Nordic waters - Fact Sheet Crepidula fornicata Author of this species fact sheet: Kathe R. Jensen, Zoological Museum, Natural History Museum of Denmark, Universiteteparken 15, 2100 København Ø, Denmark. Phone: +45 353-21083, E-mail: [email protected] Bibliographical reference – how to cite this fact sheet: Jensen, Kathe R. (2010): NOBANIS – Invasive Alien Species Fact Sheet – Crepidula fornicata – From: Identification key to marine invasive species in Nordic waters – NOBANIS www.nobanis.org, Date of access x/x/201x. Species description Species name Crepidula fornicata, Linnaeus, 1758 – Slipper limpet Synonyms Patella fornicata Linnaeus, 1758; Crepidula densata Conrad, 1871; Crepidula virginica Conrad, 1871; Crepidula maculata Rigacci, 1866; Crepidula mexicana Rigacci, 1866; Crepidula violacea Rigacci, 1866; Crepidula roseae Petuch, 1991; Crypta nautarum Mörch, 1877; Crepidula nautiloides auct. NON Lesson, 1834 (ISSG Database; Minchin, 2008). Common names Tøffelsnegl (DK, NO), Slipper limpet, American slipper limpet, Common slipper snail (UK, USA), Østerspest (NO), Ostronpest (SE), Amerikanische Pantoffelschnecke (DE), La crépidule (FR), Muiltje (NL), Seba (E). Identification Crepidula fornicata usually sit in stacks on a hard substrate, e.g., a shell of other molluscs, boulders or rocky outcroppings. Up to 13 animals have been reported in one stack, but usually 4-6 animals are seen in a stack. Individual shells are up to about 60 mm in length, cap-shaped, distinctly longer than wide. The “spire” is almost invisible, slightly skewed to the right posterior end of the shell. Shell height is highly variable. Inside the body whorl is a characteristic calcareous shell-plate (septum) behind which the visceral mass is protected. -

The American Slipper Limpet Crepidula Fornicata (L.) in the Northern Wadden Sea 70 Years After Its Introduction
Helgol Mar Res (2003) 57:27–33 DOI 10.1007/s10152-002-0119-x ORIGINAL ARTICLE D. W. Thieltges · M. Strasser · K. Reise The American slipper limpet Crepidula fornicata (L.) in the northern Wadden Sea 70 years after its introduction Received: 14 December 2001 / Accepted: 15 August 2001 / Published online: 25 September 2002 © Springer-Verlag and AWI 2002 Abstract In 1934 the American slipper limpet 1997). In the centre of its European distributional range Crepidula fornicata (L.) was first recorded in the north- a population explosion has been observed on the Atlantic ern Wadden Sea in the Sylt-Rømø basin, presumably im- coast of France, southern England and the southern ported with Dutch oysters in the preceding years. The Netherlands. This is well documented (reviewed by present account is the first investigation of the Crepidula Blanchard 1997) and sparked a variety of studies on the population since its early spread on the former oyster ecological and economic impacts of Crepidula. The eco- beds was studied in 1948. A field survey in 2000 re- logical impacts of Crepidula are manifold, and include vealed the greatest abundance of Crepidula in the inter- the following: tidal/subtidal transition zone on mussel (Mytilus edulis) (1) Accumulation of pseudofaeces and of fine sediment beds. Here, average abundance and biomass was 141 m–2 through the filtration activity of Crepidula and indi- and 30 g organic dry weight per square metre, respec- viduals protruding in stacks into the water column. tively. On tidal flats with regular and extended periods of This was reported to cause changes in sediments and emersion as well as in the subtidal with swift currents in near-bottom currents (Ehrhold et al. -

Download Preprint
1 Mobilising molluscan models and genomes in biology 2 Angus Davison1 and Maurine Neiman2 3 1. School of Life Sciences, University Park, University of Nottingham, NG7 2RD, UK 4 2. Department of Biology, University of Iowa, Iowa City, IA, USA and Department of Gender, 5 Women's, and Sexuality Studies, University of Iowa, Iowa, City, IA, USA 6 Abstract 7 Molluscs are amongst the most ancient, diverse, and important of all animal taxa. Even so, 8 no individual mollusc species has emerged as a broadly applied model system in biology. 9 We here make the case that both perceptual and methodological barriers have played a role 10 in the relative neglect of molluscs as research organisms. We then summarize the current 11 application and potential of molluscs and their genomes to address important questions in 12 animal biology, and the state of the field when it comes to the availability of resources such 13 as genome assemblies, cell lines, and other key elements necessary to mobilising the 14 development of molluscan model systems. We conclude by contending that a cohesive 15 research community that works together to elevate multiple molluscan systems to ‘model’ 16 status will create new opportunities in addressing basic and applied biological problems, 17 including general features of animal evolution. 18 Introduction 19 Molluscs are globally important as sources of food, calcium and pearls, and as vectors of 20 human disease. From an evolutionary perspective, molluscs are notable for their remarkable 21 diversity: originating over 500 million years ago, there are over 70,000 extant mollusc 22 species [1], with molluscs present in virtually every ecosystem. -

Fecundity of the Invasive Marine Gastropod Crepidula Fornicata Near the Current Northern Extreme of Its Range
Invertebrate Biology 136(4): 394–402. © 2017, The American Microscopical Society, Inc. DOI: 10.1111/ivb.12194 Fecundity of the invasive marine gastropod Crepidula fornicata near the current northern extreme of its range Jan A. Pechenik,1,a Casey M. Diederich,1 Howard I. Browman,2 and Anders Jelmert3 1 Department of Biology, Tufts University, Medford, Massachusetts 02155, USA 2 Institute of Marine Research, Austevoll Research Station, Storebø, Norway 3 Institute of Marine Research, 5817 Bergen, Norway Abstract. The calyptraeid gastropod Crepidula fornicata is native to the eastern coast of the United States but has now become an extremely successful invader along much of the Euro- pean coastline. As the northern limit of its spread is thought to be determined by an inabil- ity of adults to tolerate prolonged exposure to low winter temperatures, this study sought to compare the fecundity of females collected from two sites along the Norwegian coastline with that of females collected from Rhode Island, USA. Few other studies have compared the fecundities of marine invertebrates from invasive populations with those found in native populations. For both populations studied, fecundities increased with increasing shell length. However, contrary to expectations, size-related fecundities were significantly higher for Norwegian females than for Rhode Island females, with Norwegian females producing larger egg capsules and a greater number of embryos per capsule, but not a greater number of egg capsules per brood. Current evidence suggests that at -

Crepidula Formicatacrepidula Formicata
Crepidula formicataCrepidula formicata Easily seen Crepidula fornicata along the shoreline. Class: Gastropoda Order: Littorinimorpha Family: Calyptraeidae Genus: Crepidula Distribution It is native to the eastern This species has been introduced to several other countries, coast of North America. especially in Europe. It is considered to be an invasive, nuisance It occurs from the Gulf of species. The first known occurrence of Crepidula fornicata in St. Lawrence in the north Europe was in 1872. It was introduced accidently with imported all the way south to the American oysters. They have been repeatedly introduced ever Gulf of Mexico. since in ballast water and on ships’ hulls. Habitat They are most abundant in muddy or mixed muddy areas, This species occupies a reaching their highest densities in wave protected locations. variety of seabed surfaces. These include bays, estuaries and sheltered sides of wave- They also occur in a wide exposed islands. In Nova Scotia they are common in intertidal range of environmental and shallow subtidal regions. They attach themselves to almost conditions. any hard surface to be found (including each other and the shells of other living or dead hard-shelled invertebrates. Food They are active suspension feeders, generating a water current They live off of plankton through the mantle cavity by ciliary (hair-like) action and which they filter out of the trapping food particles on a mucous sheet lying across the front seawater. surface of the gill filament. Reproduction Being hermaphrodites they contain both male and female The Slipper limpet is a. reproductive organs. Sperm producing ability advances quicker protandric hermaphrodite. -

ABSTRACT Title of Dissertation: PATTERNS IN
ABSTRACT Title of Dissertation: PATTERNS IN DIVERSITY AND DISTRIBUTION OF BENTHIC MOLLUSCS ALONG A DEPTH GRADIENT IN THE BAHAMAS Michael Joseph Dowgiallo, Doctor of Philosophy, 2004 Dissertation directed by: Professor Marjorie L. Reaka-Kudla Department of Biology, UMCP Species richness and abundance of benthic bivalve and gastropod molluscs was determined over a depth gradient of 5 - 244 m at Lee Stocking Island, Bahamas by deploying replicate benthic collectors at five sites at 5 m, 14 m, 46 m, 153 m, and 244 m for six months beginning in December 1993. A total of 773 individual molluscs comprising at least 72 taxa were retrieved from the collectors. Analysis of the molluscan fauna that colonized the collectors showed overwhelmingly higher abundance and diversity at the 5 m, 14 m, and 46 m sites as compared to the deeper sites at 153 m and 244 m. Irradiance, temperature, and habitat heterogeneity all declined with depth, coincident with declines in the abundance and diversity of the molluscs. Herbivorous modes of feeding predominated (52%) and carnivorous modes of feeding were common (44%) over the range of depths studied at Lee Stocking Island, but mode of feeding did not change significantly over depth. One bivalve and one gastropod species showed a significant decline in body size with increasing depth. Analysis of data for 960 species of gastropod molluscs from the Western Atlantic Gastropod Database of the Academy of Natural Sciences (ANS) that have ranges including the Bahamas showed a positive correlation between body size of species of gastropods and their geographic ranges. There was also a positive correlation between depth range and the size of the geographic range. -

Table of Contents MOLLUSCA ( GASTROPODA ) Crepidula
MOLLUSCA http://www.mbl.edu/BiologicalBulletin/EGGCOMP/pages/61.html Table of Contents MOLLUSCA ( GASTROPODA ) Crepidula fornicata and C. plana The greenish-brown, boat-shaped C. fornicata adults pile one on top of another to form "chains" of individuals; the colony is attached to a stone, shell or other solid object by the bottom limpet. This species can be collected at Vineyard Haven Harbor, Mass. Another species common to the Woods Hole area, C. plana, is found within whelk or moon snail shells inhabited by large hermit crabs, and can be obtained at Cotuit. This species has a flat, whitish shell and is considerably smaller than C. fornicata. Both species are potentially protandric hermaphrodites. The active males are small, whereas the mature females are the large, older individuals; all sizes and sexual conditions can be found in any colony. C. fornicata breeds from mid-June until mid-August. C. plana has a longer season; it breeds through the first week in September (Bumpus, 1898). Sexual activity is reduced to a minimum at sea water temperatures below 15 to 16û C. (Gould, 1950). A. Care of Adults: These limpets may be kept indefinitely in aquaria or fingerbowls provided with a current of flowing, unfiltered sea water. C. plana will readily attach itself to glass when it is removed from the hermit crab shell. Unless they are already in the adult female phase, these animals eventually differentiate into males and females. B. Procuring Fertilized Ova: The animals can be detached with a heavy knife. If eggs have been deposited, they are found in a transparent capsule, attached to the substrate or to the foot of the female. -
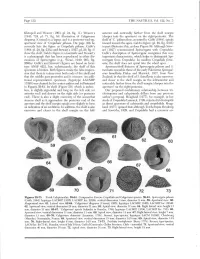
(1943: 724, Pi. 71, Fig. 16) Illustration of Calyptraea (Deeper Into the Aperture) on the Right/Posterior
Page 118 THE NAUTILUS, Vol. 122, No. 3 Kleinpeil and Weaver (1963, pi. 24, fig. 11). Weavers anterior and noticeably farther from the shell margin (1943: 724, pi. 71, fig. 16) illustration of Calyptraea (deeper into the aperture) on the right/posterior. The diegoana (Conrad) is a lapsus and is a posterior-end-up, shelf of 'C.y pileum thus, as noted by Gabb (1864), spirals apertural view of 'Crepidula' pileum. On page 356 he inward toward the apex. Gabb's figure (pi. 29, fig. 233b) correctly lists the figure as Crepidula pileum. Gabb's in part illustrates this, as does Figure 59. Although Stew- (1864: pi. 29, fig. 233a) and Stewart's (1927, pi. 29, fig. 3) art (1927) synonomized Spirocrypta with Crepidula, show the shelf. Gabb's figure is a fascimile and Stewart's Gabb's description of Spirocrypta recognizes this very is a photograph that has been reproduced in other dis- important characteristic, which helps to distinguish Spi- cussions of Spirocrypta (e.g., Wenz, 1940: 903, fig. rocrypta from Crepidula. In modern Crepidula forni- 2660a). Gabb's and Stewart's figures are based on lecto- cata, the shelf does not spiral into the whorl apex. type ANSP 4221, but, unfortunately, the shelf of this Aperture/shelf features of Spirocrypta pileum and S. specimen is broken. Both figures create the false impres- inomata resemble those of the early Paleocene Spirogal- sion that there is a sinus near both ends of the shelf and erus lamellaria Finlay and Marwick, 1937, from New that the middle part protrudes and is concave. -

Shelled Molluscs
Encyclopedia of Life Support Systems (EOLSS) Archimer http://www.ifremer.fr/docelec/ ©UNESCO-EOLSS Archive Institutionnelle de l’Ifremer Shelled Molluscs Berthou P.1, Poutiers J.M.2, Goulletquer P.1, Dao J.C.1 1 : Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France 2 : Muséum National d’Histoire Naturelle, Paris, France Abstract: Shelled molluscs are comprised of bivalves and gastropods. They are settled mainly on the continental shelf as benthic and sedentary animals due to their heavy protective shell. They can stand a wide range of environmental conditions. They are found in the whole trophic chain and are particle feeders, herbivorous, carnivorous, and predators. Exploited mollusc species are numerous. The main groups of gastropods are the whelks, conchs, abalones, tops, and turbans; and those of bivalve species are oysters, mussels, scallops, and clams. They are mainly used for food, but also for ornamental purposes, in shellcraft industries and jewelery. Consumed species are produced by fisheries and aquaculture, the latter representing 75% of the total 11.4 millions metric tons landed worldwide in 1996. Aquaculture, which mainly concerns bivalves (oysters, scallops, and mussels) relies on the simple techniques of producing juveniles, natural spat collection, and hatchery, and the fact that many species are planktivores. Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management To cite this chapter Berthou P., Poutiers J.M., Goulletquer P., Dao J.C., SHELLED MOLLUSCS, in FISHERIES AND AQUACULTURE, from Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford ,UK, [http://www.eolss.net] 1 1. -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department. -

Bivalves and Gastropods of the Gulf of Tehuantepec, Mexico: a Checklist of Species with Notes on Their Habitat and Local Distribution
Hindawi Publishing Corporation Journal of Marine Biology Volume 2009, Article ID 176801, 12 pages doi:10.1155/2009/176801 Research Article Bivalves and Gastropods of the Gulf of Tehuantepec, Mexico: A Checklist of Species with Notes on Their Habitat and Local Distribution Eduardo Rıos-Jara,´ Ceciel-M. Navarro-Caravantes, Cristian-M. Galvan-Villa,´ and Ernesto Lopez-Uriarte Laboratorio de Ecosistemas Marinos y Acuicultura, Departamento de Ecolog´ıa, Centro Universitario de Ciencias Biologicas´ y Agropecuarias, Universidad de Guadalajara, Carretera a Nogales Km. 15.5, Las Agujas Nextipac, Zapopan, Jalisco 45110, Mexico Correspondence should be addressed to Eduardo R´ıos-Jara, [email protected] Received 1 April 2009; Accepted 19 October 2009 Recommended by Ricardo Serrao˜ Santos The taxonomic composition of 160 species of bivalves and gastropods recorded in the Gulf of Tehuantepec is presented with information on their habitat and distribution along 10 different localities of the shoreline and 42 stations of the continental shelf. The species were on sandy and rocky beaches, coastal lagoons, estuaries, mangroves, rocky breakwaters of ports, and shallow subtidal areas (14–47 m depth). A total of 78 bivalve species and 82 gastropod species were recorded. Most of these were associated with sandy and rocky beaches and breakwaters of ports. The estuaries host 30 species and the coastal lagoons only two. In the shallow subtidal there were 18 gastropod species and 40 bivalve species representing 36.3% of all. This study adds 24 bivalve species and 29 gastropod species not recorded in previous studies for a total count of 213 species (102 bivalves and 111 gastropods) for Gulf of Tehuantepec. -

Effects of Conspecific Associations on Size at Sex Change in Three Species of Calyptraeid Gastropods
MARINE ECOLOGY PROGRESS SERIES Vol. 293: 89–97, 2005 Published June 2 Mar Ecol Prog Ser Effects of conspecific associations on size at sex change in three species of calyptraeid gastropods Rachel Collin1,*, Michelle McLellan1, Karl Gruber2, Catherine Bailey-Jourdain1 1Smithsonian Tropical Research Institute, Apartado Postal, 0843-03092, Balboa, Ancon, Panamá 2Bell Museum of Natural History and Department of Ecology, Evolution, and Behavior, University of Minnesota, 1987 Upper Buford Circle, St. Paul, Minnesota 55108-6097, USA ABSTRACT: In marine molluscs, sex change is often labile and is thought to be largely influenced by interactions with conspecifics. Previous studies of calyptraeid gastropods concluded that their social environment influences the timing of protandrous sex change. We conducted field surveys and labo- ratory experiments to examine the effects of conspecifics on size at sex change in 3 Panamanian calyptraeids. Crepidula cf. onyx, C. incurva Broderip, 1834 and Bostrycapulus calyptraeformis (Des- hayes, 1830) vary in densities, sex ratio and mode of development, which suggests that they might respond to associations with conspecifics in different ways. However, our laboratory experiments showed that the response to interactions with conspecifics is generally similar. In all 3 species, indi- viduals raised in isolation pass through a male phase and males raised alone change sex at the same size as males raised with another male. Both C. cf. onyx and C. incurva change sex at a larger size when kept with a female than when kept alone or with another male. The differences in size at sex change between the treatments is small and the treatment effect explains more of the variation in size at sex change in C.