An Electroencephalographic Classification for Coma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Forensic Medicine
YEREVAN STATE MEDICAL UNIVERSITY AFTER M. HERATSI DEPARTMENT OF Sh. Vardanyan K. Avagyan S. Hakobyan FORENSIC MEDICINE Handout for foreign students YEREVAN 2007 This handbook is adopted by the Methodical Council of Foreign Students of the University DEATH AND ITS CAUSES Thanatology deals with death in all its aspects. Death is of two types: (1) somatic, systemic or clinical, and (2) molecular or cellular. Somatic Death: It is the complete and irreversible stoppage of the circulation, respiration and brain functions, but there is no legal definition of death. THE MOMENT OF DEATH: Historically (medically and legally), the concept of death was that of "heart and respiration death", i.e. stoppage of spontaneous heart and breathing functions. Heart-lung bypass machines, mechanical respirators, and other devices, however have changed this medically in favor of a new concept "brain death", that is, irreversible loss of Cerebral function. Brain death is of three types: (1) Cortical or cerebral death with an intact brain stem. This produces a vegetative state in which respiration continues, but there is total loss of power of perception by the senses. This state of deep coma can be produced by cerebral hypoxia, toxic conditions or widespread brain injury. (2) Brain stem death, where the cerebrum may be intact, though cut off functionally by the stem lesion. The loss of the vital centers that control respiration, and of the ascending reticular activating system that sustains consciousness, cause the victim to be irreversibly comatose and incapable of spontaneous breathing. This can be produced by raised intracranial pressure, cerebral oedema, intracranial haemorrhage, etc.(3) Whole brain death (combination of 1 and 2). -

Glasgow Coma Scale
CalculatedPOWERED BY Decisions Clinical Decision Support for Pediatric Emergency Medicine Practice Subscribers Glasgow Coma Scale Introduction: The Glasgow Coma Scale (GCS) estimates coma severity based on eye, verbal, and motor criteria. Click the thumbnail above to access the calculator. Points & Pearls • The Glasgow Coma Scale (GCS) allows provid- agreement between providers, training and ers in multiple settings and with varying levels education are available online from the GCS of training to communicate succinctly about a creators at www.glasgowcomascale.org. patient’s mental status. • Simpler scores that have been shown to per- • The GCS has been shown to have statistical form as well as the GCS in the prehospital and correlation with a broad array of adverse neuro- emergency department setting (for initial evalu- logic outcomes, including brain injury, need for ation); these are often contracted versions of the neurosurgery, and mortality. GCS itself. For example, the Simplified Motor • The GCS score has been incorporated into Score (SMS) uses the motor portion of the GCS numerous guidelines and assessment scores only. THE SMS and other contracted scores are (eg, Advanced Cardiac Life Support, Advanced less well studied than the GCS for outcomes Trauma Life Support, Acute Physiology and like long-term mortality, and the GCS has been Chronic Health Evaluation I-III, the Trauma and studied as trended over time, while the SMS has Injury Severity Score, and the World Federation not. of Neurologic Surgeons Subarachnoid Hemor- rhage Grading Scale) Critical Actions Although it has been adopted widely and in a Points to keep in mind: variety of settings, the GCS score is not intended • Correlation with outcome and severity is most for quantitative use. -

Coma Stimulation: Suggested Activities
Coma stimulation: suggested activities Headway’s publications are all available to freely download from the information library on the charity’s website, while individuals and families can request hard copies of the booklets via the helpline. As a charity, we rely on donations from people like you to continue providing free information to people affected by brain injury. Donate today: www.headway.org.uk/donate. Introduction It is quite common for family members to feel ‘useless’ when a relative is in a coma, and to be desperate to do something to help. A coma stimulation programme (sometimes called a coma arousal programme) is an approach based on stimulating the unconscious person’s senses of hearing, touch, smell, taste and vision individually in order to help their recovery. There is still controversy over how effective it is to try to stimulate a person in coma. However, most would say that such programmes have some beneficial effect, even if only to provide something constructive for the family to do. It is very important that the activities used would have been enjoyable for the patient before the injury. For example, only play music they like and talk to them about subjects they are interested in. Try not to do anything for too long in order to avoid tiring the person out. A stimulation programme must only be started after discussion with the clinical staff, who will advise you what might be appropriate at any particular stage in the recovery process. Activity suggestions Here are some examples of activities that could form part of a coma stimulation programme: • Make sure that a few friends and family members visit regularly, rather than in large groups at a time. -

Sequential Organ Failure Assessment (SOFA) Score
CalculatedPOWERED BY Decisions Clinical Decision Support for Emergency Medicine Practice Subscribers Sequential Organ Failure Assessment (SOFA) Score Introduction: The SOFA score predicts mortality risk for patients in the intensive care unit based on lab results and clinical data. Click the thumbnail above to access the calculator. Points & Pearls • The Sequential Organ Failure Assessment namically or to determine the success or failure of (SOFA) is a mortality prediction score that is an intervention in the ICU. based on the degree of dysfunction of 6 organ systems. • The score is calculated at admission and every Why to Use 24 hours until discharge, using the worst param- The SOFA score can be used to determine the eters measured during the prior 24 hours. level of organ dysfunction and mortality risk in • The scores can be used in several ways, including: ICU patients. » As individual scores for each organ to deter- mine the progression of organ dysfunction. » As a sum of scores on a single intensive care When to Use unit (ICU) day. • The SOFA can be used on all patients who » As a sum of the worst scores during the ICU are admitted to an ICU. stay. • It is not clear whether the SOFA is reliable for • The SOFA score stratifies mortality risk in ICU patients who were transferred from another patients without restricting the data used to ICU. admission values. Critical Actions Instructions Clinical prediction scores such as the SOFA and the Calculate the SOFA score using the worst value Acute Physiologic Assessment and Chronic Health for each variable in the preceding 24-hour Evaluation (APACHE II) can be measured on all period. -

Evaluation and Management in an Urgent Care Setting
Syncope Evaluation and Management in an Urgent Care Setting Urgent message: When a patient presents to urgent care after a syncopal event, the clinician’s charge is to determine whether the episode was of benign or potentially life-threatening etiology and whether the patient should be transferred for further evaluation. Kenneth V. Iserson, MD, MBA, FACEP, FAAEM, Professor of Emergency Medicine, The University of Arizona, Tucson, AZ Introduction yncope is a sudden, transient loss of consciousness with a loss of postural tone (typically, falling). It results from an abrupt, transient, and diffuse cerebral Smalfunction and is quickly followed by sponta- neous recovery. The term syncope excludes seizures, coma, shock, or other states of altered consciousness. Many patients will ascribe their syncopal episode to a sit- uationally mediated vasovagal episode. Despite this, the goals in the urgent care setting include the following: Ⅲ Determining whether the patient’s episode was actually a syncopal or presyncopal event, and if it could have a life-threatening etiology Ⅲ Stabilizing the patient Ⅲ Transferring those patients who need further diag- nostic studies or therapeutic interventions © John Bolesky, Artville © John Bolesky, Epidemiology Syncope accounts for up to 3% of emergency depart- common in young adults, while cardiac syncope ment (ED) visits and up to 6% of hospital admissions becomes increasingly more frequent with advancing each year in the United States.1,2 At some time in their age.4 The chance of having at least one syncopal episode lives, up to about half the population (12% to 48%) of in childhood is between 15% and 50%.5 Though a people may experience syncope.3 benign cause is usually found, syncope in children war- Syncope occurs in all age groups, but it is most com- rants prompt detailed evaluation.6 mon in adults. -

Trauma Measure #5 Documentation of Glasgow Coma Score at Time of Initial Evaluation in TBI
Trauma Measure #5 Documentation of Glasgow Coma Score at Time of Initial Evaluation in TBI National Quality Strategy Domain: Communication and Care Coordination Measure Type (Process/Outcome): Process 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY DESCRIPTION: Percentage of trauma activations for patients aged 18 years or older evaluated as part of a trauma activation or trauma consultation who have a traumatic intracranial hemorrhage on initial head CT for which a Glasgow Coma Score (GCS) at the time of initial evaluation is documented in the medical record within 1 hour of hospital arrival. INSTRUCTIONS: This measure is to be reported each time a patient is evaluated as part of a trauma activation or trauma consultation and is found to have a traumatic intracranial hemorrhage on initial head CT. It is anticipated that clinicians who are responsible for the initial trauma evaluation of TBI patients as specified in the denominator coding will submit this measure. Measure Reporting via Registry: Abbreviated Injury Scale (AIS) codes, patient demographics, and the medical record are used to identify patients who are included in the measure’s denominator. The listed numerator options are used to report the numerator of the measure. DENOMINATOR: All patients aged 18 years or older evaluated by an eligible professional as part of a trauma activation or trauma consultation who have a traumatic intracranial hemorrhage on initial head CT). Denominator Criteria: Patients aged 18 years or older AND Patients evaluated as part of a trauma activation -
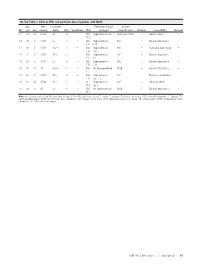
On-Line Table 1: Clinical, EEG, and Perfusion Data of Patients with NCSE Age Pre- Lat Signs/ Perfusion (Visual Seizure No
On-line Table 1: Clinical, EEG, and perfusion data of patients with NCSE Age Pre- Lat Signs/ Perfusion (visual Seizure No. (yr) Sex seizure Jerks GCS Ventilation EEG analysis) Classification Epilepsy Comorbidity Survival 1.1 59 M Coma R/Ϫ 6 ϩ SW Hyperperfusion Ins L Coma with NCSE – Cardiac arrest – FT L 1.2 72 F GTCS L/Ϫ 3 ϩ SW Hyperperfusion SSE ϩ Chronic infarction L ϩ PR PR 1.3 60 F GTCS No/Ϫ 3 ϩ SW Hyperperfusion SSE ϩ Traumatic brain injury ϩ FR FR 1.4 72 F GTCS R/ϩ 7 – SW Hyperperfusion SSE ϩ Chronic infarction L – TL TL 1.5 89 F GTCS L/Ϫ 8 – SW Hyperperfusion SSE ϩ Chronic infarction R – FR FR 1.6 67 F CP No/ϩ 11 – SW No Hyperperfusion CPSE ϩ Chronic infarction L ϩ FL 1.7 62 F GTCS R/Ϫ 5 ϩ SW Hyperperfusion SSE – Meningo-encephalitis – FR Ins,TL 1.8 67 M GTCS R/ϩ 5 ϩ SL Hyperperfusion SSE – Subacute SDH L ϩ FP L Ins L 1.9 63 F CP L/Ϫ 11 – SW No hyperperfusion CPSE ϩ Chronic infarction L ϩ TP L Note:—Lat indicates lateralizing; Pre, preceding: R, right; L, left; SW, spike-wave activity; F, frontal; T, temporal; P, parietal; Ins, insular; SDH, subdural hemorrhage; SL, slowing; EEG, electroencephalography; NCSE, nonconvulsive status epilepticus; GCS, Glasgow Coma Scale; GTCS, generalized tonic-clonic seizure; CP, complex-partial; CPSE, complex-partial status epilepticus; SSE, subtle status epilepticus. AJNR Am J Neuroradiol ͉͉www.ajnr.org E1 On-line Table 2: Clinical, EEG, and perfusion data of patients with a postictal state Age Pre- Lat Perfusion (visual No. -

The Vegetative State: Guidance on Diagnosis and Management
n CLINICAL GUIDANCE The vegetative state: guidance on diagnosis and management A report of a working party of the Royal College of Physicians contrasts with sleep, a state of eye closure and motor Clin Med 1INTRODUCTION quiescence. There are degrees of wakefulness. 2003;3:249–54 Wakefulness is normally associated with conscious awareness, but the VS indicates that wakefulness and Background awareness can be dissociated. This can occur because 1.1 This guidance has been compiled to replace the brain systems controlling wakefulness, in the the recommendations published by the Royal College upper brainstem and thalamus, are largely distinct of Physicians in 1996, 1 in response to requests for from those which mediate awareness. 6 clarification from the Official Solicitor. The guidance applies primarily to adult patients and older children Awareness in whom it is possible to apply the criteria for diagnosis discussed below. 1.6 Awareness refers to the ability to have, and the having of, experience of any kind. We are typically aware of our surroundings and of bodily sensations, Wakefulness without awareness but the contents of awareness can also include our 1.2 Consciousness is an ambiguous term, encom- memories, thoughts, emotions and intentions. passing both wakefulness and awareness. This dis- Although understanding of the brain mechanisms of tinction is crucial to the concept of the vegetative awareness is incomplete, structures in the cerebral state, in which wakefulness recovers after brain hemispheres clearly play a key role. Awareness is not injury without recovery of awareness. 2–5 a single indivisible capacity: brain damage can selectively impair some aspects of awareness, leaving others intact. -

Pediatric Index of Mortality 2 Score As an Outcome Predictor in Pediatric Intensive Care Unit in India
Research Article Pediatric index of mortality 2 score as an outcome predictor in pediatric Intensive Care Unit in India Jeyanthi Gandhi, Shanthi Sangareddi, Poovazhagi Varadarajan, Saradha Suresh Background and Aims: Pediatric index of mortality (PIM) 2 score is one of the severity Access this article online scoring systems being used for predicting outcome of patients admitted to intensive Website: www.ijccm.org care units (ICUs). The aim of the present study was to evaluate the usefulness of PIM2 DOI: 10.4103/0972-5229.120320 score in predicting mortality in a tertiary care pediatric ICU (PICU) and to assess the Quick Response Code: Abstract associated factors in predicting mortality such as presence of shock, need for assisted ventilation and Glasgow coma scale <8. Materials and Methods: This was a prospective observation study done at tertiary care PICU from May 2011 to July 2011. Consecutive 119 patients admitted to PICU (aged 1 month to 12 years) were enrolled in the study. PIM2 scoring was done for all patients. The outcome was recorded as death or discharge. The associated factors for mortality were analyzed with SPSS 17. Results: PIM2 score discriminated between death and survival at a 99.8 cut-off, with area under receiver operating characteristic curve 0.843 with 95% confi dence interval (CI) (0.765, 0.903). Most patients were referred late to this hospital, which explains higher death rate (46.2%), lesser length of hospital stay (mean 2.98 days) in the mortality group, and increased rate of mechanical ventilation (68.1%). Presence of shock was independently associated with mortality, as evidenced by binary logistic regression. -

Factors Predicting Acute Brain Injury in Cases of Carbon Monoxide Poisoning: a Prospective Registry-Based Study
toxics Article Factors Predicting Acute Brain Injury in Cases of Carbon Monoxide Poisoning: A Prospective Registry-Based Study Hoon Lim 1,†, Young Hwan Lee 1,†, Sangun Nah 1 , Sungwoo Choi 1 , Young Soon Cho 1, Gi Woon Kim 1, Ji Eun Moon 2 and Sangsoo Han 1,* 1 Department of Emergency Medicine, Soonchunhyang University Bucheon Hospital, Bucheon 14584, Korea; [email protected] (H.L.); [email protected] (Y.H.L.); [email protected] (S.N.); [email protected] (S.C.); [email protected] (Y.S.C.); [email protected] (G.W.K.) 2 Department of Biostatistics, Clinical Trial Center, Soonchunhyang University Bucheon Hospital, Bucheon 14584, Korea; [email protected] * Correspondence: [email protected] † Hoon Lim and Young Hwan Lee contributed equally to this work. Abstract: Carbon monoxide (CO) is one of the most common poisoning substances worldwide. Since acute brain injury (ABI) is an important determinant of the neurological outcome in CO poisoning, screening for patients at a high risk of developing ABI is essential for the proper treatment. This study identified predictors of ABI in patients with CO poisoning. This prospective registry-based study was conducted in patients who visited a tertiary care hospital for CO poisoning from August 2016 to June 2020. ABI was defined as the presence of acute hypoxic lesions on diffusion-weighted magnetic resonance imaging. Multiple logistic regression analysis was performed to identify the predictors of ABI. Of 231 patients, 64 (27.7%) showed ABI. Multiple logistic regression analysis showed that a Citation: Lim, H.; Lee, Y.H.; Nah, S.; Glasgow Coma Scale (GCS) score <9 at presentation (odds ratio [OR] 3.28, 95% confidence interval Choi, S.; Cho, Y.S.; Kim, G.W.; Moon, (CI) 1.08–10.01), creatinine level >1.2 mg/dL (OR 3.04, 95% CI 1.16–8.01), and C-reactive protein J.E.; Han, S. -

Predictive Value of Scoring System in Severe Pediatric Head Injury
Medicina (Kaunas) 2007; 43(11) 861 Predictive value of scoring system in severe pediatric head injury Dovilė Evalda Grinkevičiūtė, Rimantas Kėvalas, Viktoras Šaferis1, Algimantas Matukevičius1, Vytautas Ragaišis2, Arimantas Tamašauskas1 Department of Children’s Diseases, 1Institute for Biomedical Research, 2Department of Neurosurgery, Kaunas University of Medicine, Lithuania Key words: pediatric head trauma; Glasgow Coma Scale, Pediatric Trauma Score; Glasgow Outcome Scale, Pediatric Index of Mortality 2. Summary. Objectives. To determine the threshold values of Pediatric Index of Mortality 2 (PIM 2) score, Pediatric Trauma Score (PTS), and Glasgow Coma Scale (GCS) score for mortality in children after severe head injury and to evaluate changes in outcomes of children after severe head injury on discharge and after 6 months. Material and methods. All children with severe head injury admitted to the Pediatric Intensive Care Unit of Kaunas University of Medicine Hospital, Lithuania, from January 2004 to June 2006 were prospectively included in the study. The severity of head injury was categorized according to the GCS score ≤8. As initial assessment tools, the PTS, postresuscitation GCS, and PIM 2 scores were calculated for each patient. Outcome was assessed according to Glasgow Outcome Scale on discharge and after 6 months. Results. The study population consisted of 59 children with severe head injury. The group consisted of 37 (62.7%) boys and 22(37.3%) girls; the mean age was 10.6±6.02. The mean GCS, PTS, and PIM 2 scores were 5.9±1.8, 4.8±2.7, and 14.0±19.5, respectively. In terms of overall outcome, 46 (78.0%) patients survived and 13 (22.0%) died. -
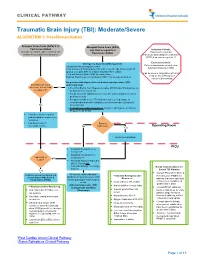
Traumatic Brain Injury (TBI): Moderate/Severe ALGORITHM 1: Post-Resuscitation
CLINICAL PATHWAY Traumatic Brain Injury (TBI): Moderate/Severe ALGORITHM 1: Post-Resuscitation Glasgow Coma Scale (GCS) 9-12 Glasgow Coma Scale (GCS) Post resuscitation less than or equal to 8 Inclusion Criteria: (Consider for children under 2 years old with Post-resuscitation Patient with traumatic concern for acute abusive head trauma) brain injury and Glasgow Coma Scale (GCS) less than or equal to 12 Exclusion Criteria: Emergency Department Management Patients found down without clear Trauma and Neurosurgery Consult traumatic brain injury (TBI) Head Computed Tomography (CT) scan- if not already obtained (STAT upload) or a quick MRI as a viable (radiation-free) option (Can be used in conjunction with Post 2 IVs with Normal Saline (NS) as maintenance Cardiac Arrest Pathway if Modified Rapid sequence intubation (RSI) if not already performed considered beneficial) For patients with signs of elevated intracranial pressure (ICP) Monitor GCS. GCS empirically treat decrease to less than Yes • 3% (0.5 mEq/mL Na+) Hypertonic saline (HTS) bolus 5mL/kg/dose via or equal to 8? peripheral IV or central line • For patients with ongoing seizures use the status epilepticus clinical pathway (below). • For patients with severe TBI who have not received a dose of levetiracetam at another institution, give levetiracetam 20mg/kg IV No (max 2 grams) • If transporting to Operating Room: Consider mild hyperventilation to PCO2 of 32-35 mmHg • Consider comfort sedation and standard neuroprotective measures. • Complete frequent Surgery neurologic exams. No Indicated?