Chapter 3 Sampling and Measuring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Check Points for Measuring Instruments
Catalog No. E12024 Check Points for Measuring Instruments Introduction Measurement… the word can mean many things. In the case of length measurement there are many kinds of measuring instrument and corresponding measuring methods. For efficient and accurate measurement, the proper usage of measuring tools and instruments is vital. Additionally, to ensure the long working life of those instruments, care in use and regular maintenance is important. We have put together this booklet to help anyone get the best use from a Mitutoyo measuring instrument for many years, and sincerely hope it will help you. CONVENTIONS USED IN THIS BOOKLET The following symbols are used in this booklet to help the user obtain reliable measurement data through correct instrument operation. correct incorrect CONTENTS Products Used for Maintenance of Measuring Instruments 1 Micrometers Digimatic Outside Micrometers (Coolant Proof Micrometers) 2 Outside Micrometers 3 Holtest Digimatic Holtest (Three-point Bore Micrometers) 4 Holtest (Two-point/Three-point Bore Micrometers) 5 Bore Gages Bore Gages 6 Bore Gages (Small Holes) 7 Calipers ABSOLUTE Coolant Proof Calipers 8 ABSOLUTE Digimatic Calipers 9 Dial Calipers 10 Vernier Calipers 11 ABSOLUTE Inside Calipers 12 Offset Centerline Calipers 13 Height Gages Digimatic Height Gages 14 ABSOLUTE Digimatic Height Gages 15 Vernier Height Gages 16 Dial Height Gages 17 Indicators Digimatic Indicators 18 Dial Indicators 19 Dial Test Indicators (Lever-operated Dial Indicators) 20 Thickness Gages 21 Gauge Blocks Rectangular Gauge Blocks 22 Products Used for Maintenance of Measuring Instruments Mitutoyo products Micrometer oil Maintenance kit for gauge blocks Lubrication and rust-prevention oil Maintenance kit for gauge Order No.207000 blocks includes all the necessary maintenance tools for removing burrs and contamination, and for applying anti-corrosion treatment after use, etc. -

Intro to Measurement Uncertainty 2011
Measurement Uncertainty and Significant Figures There is no such thing as a perfect measurement. Even doing something as simple as measuring the length of a pencil with a ruler is subject to limitations that can affect how close your measurement is to its true value. For example, you need to consider the clarity and accuracy of the scale on the ruler. What is the smallest subdivision of the ruler scale? How thick are the black lines that indicate centimeters and millimeters? Such ideas are important in understanding the limitations of use of a ruler, just as with any measuring device. The degree to which a measured quantity compares to the true value of the measurement describes the accuracy of the measurement. Most measuring instruments you will use in physics lab are quite accurate when used properly. However, even when an instrument is used properly, it is quite normal for different people to get slightly different values when measuring the same quantity. When using a ruler, perception of when an object is best lined up against the ruler scale may vary from person to person. Sometimes a measurement must be taken under less than ideal conditions, such as at an awkward angle or against a rough surface. As a result, if the measurement is repeated by different people (or even by the same person) the measured value can vary slightly. The degree to which repeated measurements of the same quantity differ describes the precision of the measurement. Because of limitations both in the accuracy and precision of measurements, you can never expect to be able to make an exact measurement. -

The Gage Block Handbook
AlllQM bSflim PUBUCATIONS NIST Monograph 180 The Gage Block Handbook Ted Doiron and John S. Beers United States Department of Commerce Technology Administration NET National Institute of Standards and Technology The National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. Major technical operating units and their principal -

Surftest SJ-400
Form Measurement Surftest SJ-400 Bulletin No. 2013 Aurora, Illinois (Corporate Headquarters) (630) 978-5385 Westford, Massachusetts (978) 692-8765 Portable Surface Roughness Tester Huntersville, North Carolina (704) 875-8332 Mason, Ohio (513) 754-0709 Plymouth, Michigan (734) 459-2810 City of Industry, California (626) 961-9661 Kirkland, Washington (408) 396-4428 Surftest SJ-400 Series Revolutionary New Portable Surface Roughness Testers Make Their Debut Long-awaited performance and functionality are here: compact design, skidless and high-accuracy roughness measurements, multi-functionality and ease of operation. Requirement Requirement High-accuracy1 measurements with 3 Cylinder surface roughness measurements with a hand-held tester a hand-held tester A wide range, high-resolution detector and an ultra-straight drive The skidless measurement and R-surface compensation functions unit provide class-leading accuracy. make it possible to evaluate cylinder surface roughness. Detector Measuring range: 800µm Resolution: 0.000125µm (on 8µm range) Drive unit Straightness/traverse length SJ-401: 0.3µm/.98"(25mm) SJ-402: 0.5µm/1.96"(50mm) SJ-401 SJ-402 SJ-401 Requirement Requirement Roughness parameters2 that conform to international standards The SJ-400 Series can evaluate 36 kinds of roughness parameters conforming to the latest ISO, DIN, and ANSI standards, as well as to JIS standards (1994/1982). 4 Measurement/evaluation of stepped features and straightness Ultra-fine steps, straightness and waviness are easily measured by switching to skidless measurement mode. The ruler function enables simpler surface feature evaluation on the LCD monitor. 2 3 Requirement Measurement Applications R-surface 5 measurement Advanced data processing with extended analysis The SJ-400 Series allows data processing identical to that in the high-end class. -

Length Scale Measurement Procedures at the National Bureau of 1 Standards
NEW NIST PUBLICATION June 12, 1989 NBSIR 87-3625 Length Scale Measurement Procedures at the National Bureau of 1 Standards John S. Beers, Guest Worker U.S. DEPARTMENT OF COMMERCE National Bureau of Standards National Engineering Laboratory Precision Engineering Division Gaithersburg, MD 20899 September 1987 U.S. DEPARTMENT OF COMMERCE NBSIR 87-3625 LENGTH SCALE MEASUREMENT PROCEDURES AT THE NATIONAL BUREAU OF STANDARDS John S. Beers, Guest Worker U.S. DEPARTMENT OF COMMERCE National Bureau of Standards National Engineering Laboratory Precision Engineering Division Gaithersburg, MD 20899 September 1987 U.S. DEPARTMENT OF COMMERCE, Clarence J. Brown, Acting Secretary NATIONAL BUREAU OF STANDARDS, Ernest Ambler, Director LENGTH SCALE MEASUREMENT PROCEDURES AT NBS CONTENTS Page No Introduction 1 1.1 Purpose 1 1.2 Line scales of length 1 1.3 Line scale calibration and measurement assurance at NBS 2 The NBS Line Scale Interferometer 2 2.1 Evolution of the instrument 2 2.2 Original design and operation 3 2.3 Present form and operation 8 2.3.1 Microscope system electronics 8 2.3.2 Interferometer 9 2.3.3 Computer control and automation 11 2.4 Environmental measurements 11 2.4.1 Temperature measurement and control 13 2.4.2 Atmospheric pressure measurement 15 2.4.3 Water vapor measurement and control 15 2.5 Length computations 15 2.5.1 Fringe multiplier 16 2.5.2 Analysis of redundant measurements 17 3. Measurement procedure 18 Planning and preparation 18 Evaluation of the scale 18 Calibration plan 19 Comparator preparation 20 Scale preparation, -

Viscoelastic Behavior of Foamed Polystyrene/Paper Composites
Session 2793 Viscoelastic Behavior of Foamed Polystyrene/Paper Composites Robert A. McCoy Youngstown State University Introduction This paper outlines a simple lab experiment for high school students or freshman engineering students designed to demonstrate the principle behind why sandwich composites are so stiff as well as light-weight. A sandwich composite consists of a very lightweight core (such as a foamed polymer or honeycomb structure) with sheets of another material (such as paper, plastic, fiberglass, or aluminum) on the top and bottom surfaces. Applications for sandwich composites requiring both high-stiffness and lightweight include aircraft panels, boat hulls, jet skis, snow skis, partitions, and garage doors. In this experiment, the students measure the increase in stiffness when the top and bottom skins of paper are added to a Styrofoam beam to form the sandwich composite. Also this experiment includes a creep test in which the students measure and plot the deflection of the Styrofoam beam versus time to illustrate the viscoelastic behavior of Styrofoam. Materials and Equipment Required 1. A sheet of Styrofoam (foamed polystyrene) approximately 122 cm (4 ft) long, 35.5 cm (14 in) wide, and 1.83 cm (0.72 in) thick. 2. A metal frame with a clamp to hold one end of the Styrofoam beam. 3. Six metal washers, about 3.8 cm (1.5 in) diameter. 4. One jumbo paperclip. 5. One large paper grocery bag. 6. Scissors, cutting knife, and paper glue. 7. A ruler or meter stick. 8. A weighing scale or balance 9. A computer with MS Excel Procedure For each group of students performing this experiment, at least four rectangular bars approximately 2.5 cm (1 in) wide and 35.5 cm (14 in) long were cut from the Styrofoam sheet. -

Weighing Scale 1 Weighing Scale
Weighing scale 1 Weighing scale A weighing scale (usually just "scales" in UK and Australian English, "weighing machine" in south Asian English or "scale" in US English) is a measuring instrument for determining the weight or mass of an object. A spring scale measures weight by the distance a spring deflects under its load. A balance compares the torque on the arm due to the sample weight to the torque on the arm due to a standard reference weight using a horizontal lever. Balances are different from scales, in that a balance measures mass (or more specifically gravitational mass), whereas a scale measures weight (or more specifically, either the tension or compression force of constraint provided by the scale). Weighing scales are used in many industrial and commercial applications, and products from feathers to loaded tractor-trailers are sold by weight. Specialized medical scales and bathroom scales are used to measure the body weight of human beings. History Emperor Jahangir (reign 1605 - 1627) weighing The balance scale is such a simple device that its usage likely far his son Shah Jahan on a weighing scale by artist predates the evidence. What has allowed archaeologists to link artifacts Manohar (AD 1615, Mughal dynasty, India). to weighing scales are the stones for determining absolute weight. The balance scale itself was probably used to determine relative weight long before absolute weight.[1] The oldest evidence for the existence of weighing scales dates to c. 2400-1800 B.C.E. in the Indus River valley (modern-day Pakistan). Uniform, polished stone cubes discovered in early settlements were probably used as weight-setting stones in balance scales. -

Quick Guide to Precision Measuring Instruments
E4329 Quick Guide to Precision Measuring Instruments Coordinate Measuring Machines Vision Measuring Systems Form Measurement Optical Measuring Sensor Systems Test Equipment and Seismometers Digital Scale and DRO Systems Small Tool Instruments and Data Management Quick Guide to Precision Measuring Instruments Quick Guide to Precision Measuring Instruments 2 CONTENTS Meaning of Symbols 4 Conformance to CE Marking 5 Micrometers 6 Micrometer Heads 10 Internal Micrometers 14 Calipers 16 Height Gages 18 Dial Indicators/Dial Test Indicators 20 Gauge Blocks 24 Laser Scan Micrometers and Laser Indicators 26 Linear Gages 28 Linear Scales 30 Profile Projectors 32 Microscopes 34 Vision Measuring Machines 36 Surftest (Surface Roughness Testers) 38 Contracer (Contour Measuring Instruments) 40 Roundtest (Roundness Measuring Instruments) 42 Hardness Testing Machines 44 Vibration Measuring Instruments 46 Seismic Observation Equipment 48 Coordinate Measuring Machines 50 3 Quick Guide to Precision Measuring Instruments Quick Guide to Precision Measuring Instruments Meaning of Symbols ABSOLUTE Linear Encoder Mitutoyo's technology has realized the absolute position method (absolute method). With this method, you do not have to reset the system to zero after turning it off and then turning it on. The position information recorded on the scale is read every time. The following three types of absolute encoders are available: electrostatic capacitance model, electromagnetic induction model and model combining the electrostatic capacitance and optical methods. These encoders are widely used in a variety of measuring instruments as the length measuring system that can generate highly reliable measurement data. Advantages: 1. No count error occurs even if you move the slider or spindle extremely rapidly. 2. You do not have to reset the system to zero when turning on the system after turning it off*1. -
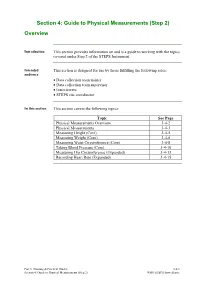
Section 4: Guide to Physical Measurements (Step 2) Overview
Section 4: Guide to Physical Measurements (Step 2) Overview Introduction This section provides information on and is a guide to working with the topics covered under Step 2 of the STEPS Instrument. Intended This section is designed for use by those fulfilling the following roles: audience • Data collection team trainer • Data collection team supervisor • Interviewers • STEPS site coordinator In this section This section covers the following topics: Topic See Page Physical Measurements Overview 3-4-2 Physical Measurements 3-4-3 Measuring Height (Core) 3-4-5 Measuring Weight (Core) 3-4-6 Measuring Waist Circumference (Core) 3-4-8 Taking Blood Pressure (Core) 3-4-10 Measuring Hip Circumference (Expanded) 3-4-13 Recording Heart Rate (Expanded) 3-4-15 Part 3: Training & Practical Guides 3-4-1 Section 4: Guide to Physical Measurements (Step 2) WHO STEPS Surveillance Physical Measurements Overview Introduction Step 2 of the STEPS Instrument includes the addition of selected physical measures to determine the proportion of adults that: • Are overweight and/or obese • Have raised blood pressure What you will In this section, you will learn: learn • What the physical measures are and what they mean • What equipment you will need • How to assemble and use the equipment • How to take physical measurements and accurately record the results Learning The learning outcome of this section is to understand what the physical outcomes measures are and how to accurately take the measurements and record the objectives results. Part 3: Training & Practical Guides 3-4-2 Section 4: Guide to Physical Measurements (Step 2) WHO STEPS Surveillance Physical Measurements Introduction Height and weight measurements are taken from eligible participants to calculate body mass index (BMI) used to determine overweight and obesity. -

Part Ii: Procedures and Equipment for Weighing, Measuring and Recording Anthropometric Data
PART II: PROCEDURES AND EQUIPMENT FOR WEIGHING, MEASURING AND RECORDING ANTHROPOMETRIC DATA In order to accurately reflect health status, measurements must be taken carefully using standardized techniques. The results must be recorded accurately and compared with the appropriate references. Measurements do not need to be done twice, if the one measurement is done correctly, using proper equipment and technique. If there is any doubt about the accuracy of a measurement, it should be repeated. Measuring Recumbent Length of Infants and Young Children Until age 2, children must be measured in the recumbent position. Between 2 and 3 years of age the CPA (or individual measuring the child) must decide whether a recumbent or standing measurement is most appropriate. If the child is <32”, a recumbent measurement must be used, because the charts based on standing height apply only to heights > 32”. For children 2 to 3 years of age, taller than 32”, the best guideline is to think about the physical abilities of the child. Generally, if a child can stand unassisted and follow directions for proper positioning, a standing measure should be taken. If the child cannot stand straight, a recumbent length should be measured. birth to 24 months………measure recumbent length . between 2 and 3 years of age: o if < 32”……………….measure recumbent length o if ≥ 32” if child can stand unassisted in proper position, measure standing if child cannot stand properly, measure recumbent length . over 3 years of age……………..measure standing height A. Equipment: Use a recumbent measuring board with a fixed headpiece and sliding foot piece that are both perpendicular/upright (form a 90-degree angle) to the measurement surface. -

I Introduction to Measurement and Data Analysis
I Introduction to Measurement and Data Analysis Introduction to Measurement In physics lab the activity in which you will most frequently be engaged is measuring things. Using a wide variety of measuring instruments you will measure times, temperatures, masses, forces, speeds, frequencies, energies, and many more physical quantities. Your tools will span a range of technologies from the simple (such as a ruler) to the complex (perhaps a digital computer). Certainly it would be worthwhile to devote a little time and thought to some of the details of \measuring things" that may have not yet occurred to you. True Value - How Tall? At first thought you might suppose that the goal of measurement is a very straightforward one: find the true value of the thing being measured. Alas, things are seldom as simple as we would like. Consider the following \case study." Suppose you wished to measure how your lab partner's height. One way might be to simply look at him or her and estimate, \Oh, about five-nine," meaning five feet, nine inches tall. Of course you couldn't be sure that five-eight or five-ten, or even five-eleven might be a better estimate. In other words, your measurement (estimate) is uncertain by some amount, perhaps an inch or two either way. The \true value" lies somewhere within a range of uncertainty and one way to express this notion is to say that your partner's height is five feet, nine inches plus or minus two inches or 69 ± 2 inches. It should begin to be clear that at least one of the goals of measurement is to reduce the uncertainty to as small an amount as is feasible and useful. -

Comparison of Internal and External Threads Pitch Diameter Measurement by Using Conventional Methods and CMM’S
19th International Congress of Metrology, 09001 (2019) https://doi.org/10.1051/metrology/201909001 Comparison of internal and external threads pitch diameter measurement by using conventional methods and CMM’s İ. Ahmet Yüksel1*, T. Oytun Kılınç1, K. Berk Sönmez1, and Sinem Ön Aktan1 1Department of Laboratories and Calibration Management, Roketsan Missiles Industries Inc., 06780 Ankara, Turkey Abstract. Threads are very frequent and critical connection methods of components to build final products as per desired technical requirements. As it is used for very wide numbers of applications and industries, tolerances and thread structure differ as per requirements of applied areas. After theoretical decision of threaded parts, one hard task is to manufacture this thread as required. Correct flank angle, pitch diameter, pitch, height, major/minor diameters shall be obtained to receive desired mechanical connection properties. So as a result of all importance of threaded connection, it is also important to control thread properties. In this study it is aimed to show that as a result of significant development on sensor and probing technology, Coordinate Measuring Machines (CMMs) are good alternatives of conventional methods by considering accuracy, time requirement, setup requirements, and ease of application. In order to evaluate competence of regular bridge type CMMs regarding simple pitch diameter measurement, results of pitch diameter from symmetric thread with 30º flank angles will be taken by 1D length measurement device and bridge type CMM to compare. Comparison of conventional methods and CMM’s result shows that CMMs also provide accurate and precise results and can be used for calibration of gages as an alternative method with a number of advantages which are shown by this study.