ID-77: Nut Tree Growing in Kentucky
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHESTNUT (CASTANEA Spp.) CULTIVAR EVALUATION for COMMERCIAL CHESTNUT PRODUCTION
CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE By Ana Maria Metaxas Approved: James Hill Craddock Jennifer Boyd Professor of Biological Sciences Assistant Professor of Biological and Environmental Sciences (Director of Thesis) (Committee Member) Gregory Reighard Jeffery Elwell Professor of Horticulture Dean, College of Arts and Sciences (Committee Member) A. Jerald Ainsworth Dean of the Graduate School CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE by Ana Maria Metaxas A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements for the Degree of Master of Science in Environmental Science May 2013 ii ABSTRACT Chestnut cultivars were evaluated for their commercial applicability under the environmental conditions in Hamilton County, TN at 35°13ꞌ 45ꞌꞌ N 85° 00ꞌ 03.97ꞌꞌ W elevation 230 meters. In 2003 and 2004, 534 trees were planted, representing 64 different cultivars, varieties, and species. Twenty trees from each of 20 different cultivars were planted as five-tree plots in a randomized complete block design in four blocks of 100 trees each, amounting to 400 trees. The remaining 44 chestnut cultivars, varieties, and species served as a germplasm collection. These were planted in guard rows surrounding the four blocks in completely randomized, single-tree plots. In the analysis, we investigated our collection predominantly with the aim to: 1) discover the degree of acclimation of grower- recommended cultivars to southeastern Tennessee climatic conditions and 2) ascertain the cultivars’ ability to survive in the area with Cryphonectria parasitica and other chestnut diseases and pests present. -

Natural Colourants with Ancient Concept and Probable Uses
JOURNAL OF ADVANCED BOTANY AND ZOOLOGY Journal homepage: http://scienceq.org/Journals/JABZ.php Review Open Access Natural Colourants With Ancient Concept and Probable Uses Tabassum Khair1, Sujoy Bhusan2, Koushik Choudhury2, Ratna Choudhury3, Manabendra Debnath4 and Biplab De2* 1 Department of Pharmaceutical Sciences, Assam University, Silchar, Assam, India. 2 Regional Institute of Pharmaceutical Science And Technology, Abhoynagar, Agartala, Tripura, India. 3 Rajnagar H. S. School, Agartala, Tripura, India. 4 Department of Human Physiology, Swami Vivekananda Mahavidyalaya, Mohanpur, Tripura, India. *Corresponding author: Biplab De, E-mail: [email protected] Received: February 20, 2017, Accepted: April 15, 2017, Published: April 15, 2017. ABSTRACT: The majority of natural colourants are of vegetable origin from plant sources –roots, berries, barks, leaves, wood and other organic sources such as fungi and lichens. In the medicinal and food products apart from active constituents there are several other ingredients present which are used for either ethical or technical reasons. Colouring agent is one of them, known as excipients. The discovery of man-made synthetic dye in the mid-19th century triggered a long decline in the large-scale market for natural dyes as practiced by the villagers and tribes. The continuous use of synthetic colours in textile and food industry has been found to be detrimental to human health, also leading to environmental degradation. Biocolours are extracted by the villagers and certain tribes from natural herbs, plants as leaves, fruits (rind or seeds), flowers (petals, stamens), bark or roots, minerals such as prussian blue, red ochre & ultramarine blue and are also of insect origin such as lac, cochineal and kermes. -
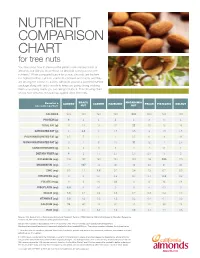
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Erigenia : Journal of the Southern Illinois Native Plant Society
ERIGENIA THE LIBRARY OF THE DEC IS ba* Number 13 UNIVERSITY OF ILLINOIS June 1994 ^:^;-:A-i.,-CS..;.iF/uGN SURVEY Conference Proceedings 26-27 September 1992 Journal of the Eastern Illinois University Illinois Native Plant Society Charleston Erigenia Number 13, June 1994 Editor: Elizabeth L. Shimp, U.S.D.A. Forest Service, Shawnee National Forest, 901 S. Commercial St., Harrisburg, IL 62946 Copy Editor: Floyd A. Swink, The Morton Arboretum, Lisle, IL 60532 Publications Committee: John E. Ebinger, Botany Department, Eastern Illinois University, Charleston, IL 61920 Ken Konsis, Forest Glen Preserve, R.R. 1 Box 495 A, Westville, IL 61883 Kenneth R. Robertson, Illinois Natural History Survey, 607 E. Peabody Dr., Champaign, IL 61820 Lawrence R. Stritch, U.S.D.A. Forest Service, Shawnee National Forest, 901 S. Commercial Su, Harrisburg, IL 62946 Cover Design: Christopher J. Whelan, The Morton Arboretum, Lisle, IL 60532 Cover Illustration: Jean Eglinton, 2202 Hazel Dell Rd., Springfield, IL 62703 Erigenia Artist: Nancy Hart-Stieber, The Morton Arboretum, Lisle, IL 60532 Executive Committee of the Society - April 1992 to May 1993 President: Kenneth R. Robertson, Illinois Natural History Survey, 607 E. Peabody Dr., Champaign, IL 61820 President-Elect: J. William Hammel, Illinois Environmental Protection Agency, Springfield, IL 62701 Past President: Jon J. Duerr, Kane County Forest Preserve District, 719 Batavia Ave., Geneva, IL 60134 Treasurer: Mary Susan Moulder, 918 W. Woodlawn, Danville, IL 61832 Recording Secretary: Russell R. Kirt, College of DuPage, Glen EUyn, IL 60137 Corresponding Secretary: John E. Schwegman, Illinois Department of Conservation, Springfield, IL 62701 Membership: Lorna J. Konsis, Forest Glen Preserve, R.R. -

Native Trees of Georgia
1 NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION Eleventh Printing - 2001 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W. -

Scientific Name Common Name NATURAL ASSOCIATIONS of TREES and SHRUBS for the PIEDMONT a List
www.rainscapes.org NATURAL ASSOCIATIONS OF TREES AND SHRUBS FOR THE PIEDMONT A list of plants which are naturally found growing with each other and which adapted to the similar growing conditions to each other Scientific Name Common Name Acer buergeranum Trident maple Acer saccarum Sugar maple Acer rubrum Red Maple Betula nigra River birch Trees Cornus florida Flowering dogwood Fagus grandifolia American beech Maple Woods Liriodendron tulipifera Tulip-tree, yellow poplar Liquidamber styraciflua Sweetgum Magnolia grandiflora Southern magnolia Amelanchier arborea Juneberry, Shadbush, Servicetree Hamamelis virginiana Autumn Witchhazel Shrubs Ilex opaca American holly Ilex vomitoria*** Yaupon Holly Viburnum acerifolium Maple leaf viburnum Aesulus parvilflora Bottlebrush buckeye Aesulus pavia Red buckeye Carya ovata Shadbark hickory Cornus florida Flowering dogwood Halesia carolina Crolina silverbell Ilex cassine Cassina, Dahoon Ilex opaca American Holly Liriodendron tulipifera Tulip-tree, yellow poplar Trees Ostrya virginiana Ironwood Prunus serotina Wild black cherry Quercus alba While oak Quercus coccinea Scarlet oak Oak Woods Quercus falcata Spanish red oak Quercus palustris Pin oak Quercus rubra Red oak Quercus velutina Black oak Sassafras albidum Sassafras Azalea nudiflorum Pinxterbloom azalea Azalea canescens Piedmont azalea Ilex verticillata Winterberry Kalmia latifolia Mountain laurel Shrubs Rhododenron calendulaceum Flame azalea Rhus copallina Staghorn sumac Rhus typhina Shining sumac Vaccinium pensylvanicum Low-bush blueberry Magnolia -
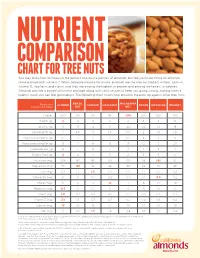
Chart for Tree Nuts
NUTRIENT COMPARISON CHART FOR TREE NUTS You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the highest in protein and among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a healthy snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT Calories 1602 190 160 180 200 200 160 190 Protein (g) 6 4 4 4 2 3 6 4 Total Fat (g) 14 19 13 17 22 20 13 18 Saturated Fat (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 Polyunsaturated Fat (g) 3.5 7 2 2 0.5 6 4 13 Monounsaturated Fat (g) 9 7 8 13 17 12 7 2.5 Carbohydrates (g) 6 3 9 5 4 4 8 4 Dietary Fiber (g) 4 2 1 3 2 3 3 2 Potassium (mg) 208 187 160 193 103 116 285 125 Magnesium (mg) 77 107 74 46 33 34 31 45 Zinc (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 Vitamin B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 Folate (mcg) 12 6 20 32 3 6 14 28 Riboflavin (mg) 0.3 0 0.1 0 0 0 0.1 0 Niacin (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 Vitamin E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.6 0.2 Calcium (mg) 76 45 13 32 20 20 30 28 Iron (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Conservation and Management of Butternut Trees
Purdue University Purdue extension FNR-421-W & Natural Re ry sou Forestry and Natural Resources st rc re e o s F Conservation and Management of Butternut Trees Lenny Farlee1,3, Keith Woeste1, Michael Ostry2, James McKenna1 and Sally Weeks3 1 USDA Forest Service Hardwood Tree Improvement and Regeneration Center, Purdue University, 715 W. State Street, West Lafayette, IN, 47907 PURDUE UNIVERSITY 2 USDA Forest Service Northern Research Station, 1561 Lindig Ave. St. Paul, MN 55108 3 Department of Forestry and Natural Resources, Purdue University, 715 W. State Street, West Lafayette, IN, 47907 Introduction Butternut (Juglans cinerea), also known as white wal- nut, is a native hardwood related to black walnut (Juglans nigra) and other members of the walnut family. Butternut is a medium-sized tree with alternate, pinnately com- pound leaves, that bears large, sharply ridged, cylindrical nuts inside sticky green hulls that earned it the nickname lemon-nut (Rink, 1990). The nuts, a preferred food of squirrels and other wildlife, were collected and eaten by Native Americans (Waugh, 1916; Hamel and Chiltoskey, 1975) and early settlers, who also valued butternut for its workable, medium brown-colored heartwood (Kel- logg, 1919), and as a source of medicine (Johnson, 1884; Lawrence, 1998), dyes (Hamel and Chiltoskey, 1975), and sap sugar. Butternut’s native range extends over the entire north- eastern quarter of the United States, including many states immediately west of the Mississippi River. Butter- nut is more cold-tolerant than black walnut, and it grows as far north as the Upper Peninsula of Michigan, New Brunswick, southern Quebec, and Ontario (Fig.1). -

Conservation Assessment for Butternut Or White Walnut (Juglans Cinerea) L. USDA Forest Service, Eastern Region
Conservation Assessment for Butternut or White walnut (Juglans cinerea) L. USDA Forest Service, Eastern Region 2003 Jan Schultz Hiawatha National Forest Forest Plant Ecologist (906) 228-8491 This Conservation Assessment was prepared to compile the published and unpublished information on Juglans cinerea L. (butternut). This is an administrative review of existing information only and does not represent a management decision or direction by the U. S. Forest Service. Though the best scientific information available was gathered and reported in preparation of this document, then subsequently reviewed by subject experts, it is expected that new information will arise. In the spirit of continuous learning and adaptive management, if the reader has information that will assist in conserving the subject taxon, please contact the Eastern Region of the Forest Service Threatened and Endangered Species Program at 310 Wisconsin Avenue, Milwaukee, Wisconsin 53203. Conservation Assessment for Butternut or White walnut (Juglans cinerea) L. 2 Table Of Contents EXECUTIVE SUMMARY .....................................................................................5 INTRODUCTION / OBJECTIVES.......................................................................7 BIOLOGICAL AND GEOGRAPHICAL INFORMATION..............................8 Species Description and Life History..........................................................................................8 SPECIES CHARACTERISTICS...........................................................................9 -

Juglans Nigra Juglandaceae L
Juglans nigra L. Juglandaceae LOCAL NAMES English (walnut,American walnut,eastern black walnut,black walnut); French (noyer noir); German (schwarze Walnuß); Portuguese (nogueira- preta); Spanish (nogal negro,nogal Americano) BOTANIC DESCRIPTION Black walnut is a deciduous tree that grows to a height of 46 m but ordinarily grows to around 25 m and up to 102 cm dbh. Black walnut develops a long, smooth trunk and a small rounded crown. In the open, the trunk forks low with a few ascending and spreading coarse branches. (Robert H. Mohlenbrock. USDA NRCS. The root system usually consists of a deep taproot and several wide- 1995. Northeast wetland flora: Field office spreading lateral roots. guide to plant species) Leaves alternate, pinnately compound, 30-70 cm long, up to 23 leaflets, leaflets are up to 13 cm long, serrated, dark green with a yellow fall colour in autumn and emits a pleasant sweet though resinous smell when crushed or bruised. Flowers monoecious, male flowers catkins, small scaley, cone-like buds; female flowers up to 8-flowered spikes. Fruit a drupe-like nut surrounded by a fleshy, indehiscent exocarp. The nut has a rough, furrowed, hard shell that protects the edible seed. Fruits Bark (Robert H. Mohlenbrock. USDA NRCS. 1995. Northeast wetland flora: Field office produced in clusters of 2-3 and borne on the terminals of the current guide to plant species) season's growth. The seed is sweet, oily and high in protein. The bitter tasting bark on young trees is dark and scaly becoming darker with rounded intersecting ridges on maturity. BIOLOGY Flowers begin to appear mid-April in the south and progressively later until early June in the northern part of the natural range. -

Filbert European Corylus Avellana Corylus Avellana Commonly Called
Filbert European Corylus avellana Corylus avellana commonly called European Filbert, European hazel, cobnut and Harry Lauder’s walking stick is a deciduous, thicket-forming, multi-trunked suckering shrub. Common names of filbert and hazel are likely interchangeable. Hazel is more often used in reference to wild specimens and filbert is more likely to be used in reference to cultivated plants. The filbert nuts to be produced in commerce primarily come from plants (C. avellano x C. maxima). ‘Contorta’, commonly called contorted filbert, corkscrew hazel or Harry Lauder’s Walking Stick, is contorted version of the species plant. It was discovered growing as a sport in an English hedgerow In the mid-1800s by Victorian Gardner Cannon Ellacombe. This plant was given the common name of Harry Lauder’s walking stick in the 1900s in honor of the Scottish entertainer Harry Lauder. The European Filbert leaves are dark green, slightly covered with fine soft hairs above and beneath; alternate; 2-4” in length, somewhat circular to egg – shaped or heart – shaped, abruptly tapers to a point at apex, edge doubly toothed, often with lobes, petiole ¼” to ½” long. The twigs are brown, glandular – hairy. Buds green to brown, hairless with hairy scale; overlapping, egg shaped to round. Flowers/Fruit: Flowers monoecious; male flowers are large (2”to 3”) catkins, yellow – brown, late winter to early spring blooming; female flowers inconspicuous. Fruit a nut; nuts inside involucre, which is toothed or lubed and nearly the length of the nut; ¾” in length; edible fruit grown commercially as a crop. European Filbert bark is pale to gray – brown, smoother with age, not an ornamental feature. -

Nutritive Value and Degradability of Leaves from Temperate Woody Resources for Feeding Ruminants in Summer
3rd European Agroforestry Conference Montpellier, 23-25 May 2016 Silvopastoralism (poster) NUTRITIVE VALUE AND DEGRADABILITY OF LEAVES FROM TEMPERATE WOODY RESOURCES FOR FEEDING RUMINANTS IN SUMMER Emile JC 1*, Delagarde R 2, Barre P 3, Novak S 1 Corresponding author: [email protected] mailto:(1) INRA, UE 1373 FERLUS, 86600 Lusignan, France (2) INRA, UMR 1348 INRA-Agrocampus Ouest, 35590 Saint-Gilles, France (3) INRA, UR 4 URP3F, 86600 Lusignan, France 1/ Introduction Integrating agroforestry in livestock farming systems may be a real opportunity in the current climatic, social and economic conditions. Trees can contribute to improve welfare of grazing ruminants. The production of leaves from woody plants may also constitute a forage resource for livestock (Papanastasis et al. 2008) during periods of low grasslands production (summer and autumn). To know the potential of leaves from woody plants to be fed by ruminants, including dairy females, the nutritive value of these new forages has to be evaluated. References on nutritive values that already exist for woody plants come mainly from tropical or Mediterranean climatic conditions (http://www.feedipedia.org/) and very few data are currently available for the temperate regions. In the frame of a long term mixed crop-dairy system experiment integrating agroforestry (Novak et al. 2016), a large evaluation of leaves from woody resources has been initiated. The objective of this evaluation is to characterise leaves of woody forage resources potentially available for ruminants (hedgerows, coppices, shrubs, pollarded trees), either directly by browsing or fed after cutting. This paper presents the evaluation of a first set of 12 woody resources for which the feeding value is evaluated through their protein and fibre concentrations, in vitro digestibility (enzymatic method) and effective ruminal degradability.