Frontal Operculum Gliomas: Language Outcome Following Resection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Lesions in the Right Rolandic Operculum Are Associated with Self
www.nature.com/scientificreports OPEN Lesions in the right Rolandic operculum are associated with self‑rating afective and apathetic depressive symptoms for post‑stroke patients Stephanie Sutoko1,2*, Hirokazu Atsumori1,2, Akiko Obata1,2, Tsukasa Funane1,2, Akihiko Kandori1,2, Koji Shimonaga3,4, Seiji Hama2,4, Shigeto Yamawaki5 & Toshio Tsuji6 Stroke survivors majorly sufered from post‑stroke depression (PSD). The PSD diagnosis is commonly performed based on the clinical cut‑of for psychometric inventories. However, we hypothesized that PSD involves spectrum symptoms (e.g., apathy, depression, anxiety, and stress domains) and severity levels. Therefore, instead of using the clinical cut‑of, we suggested a data‑driven analysis to interpret patient spectrum conditions. The patients’ psychological conditions were categorized in an unsupervised manner using the k‑means clustering method, and the relationships between psychological conditions and quantitative lesion degrees were evaluated. This study involved one hundred sixty‑fve patient data; all patients were able to understand and perform self‑rating psychological conditions (i.e., no aphasia). Four severity levels—low, low‑to‑moderate, moderate‑ to‑high, and high—were observed for each combination of two psychological domains. Patients with worse conditions showed the signifcantly greater lesion degree at the right Rolandic operculum (part of Brodmann area 43). The dissimilarities between stress and other domains were also suggested. Patients with high stress were specifcally associated with lesions in the left thalamus. Impaired emotion processing and stress‑afected functions have been frequently related to those lesion regions. Those lesions were also robust and localized, suggesting the possibility of an objective for predicting psychological conditions from brain lesions. -

Surgical Anatomy of the Insula Christophe Destrieux, Igor Lima Maldonado, Louis-Marie Terrier, Ilyess Zemmoura
Surgical Anatomy of the Insula Christophe Destrieux, Igor Lima Maldonado, Louis-Marie Terrier, Ilyess Zemmoura To cite this version: Christophe Destrieux, Igor Lima Maldonado, Louis-Marie Terrier, Ilyess Zemmoura. Surgical Anatomy of the Insula. Mehmet Turgut; Canan Yurttaş; R. Shane Tubbs. Island of Reil (Insula) in the Human Brain. Anatomical, Functional, Clinical and Surgical Aspects, 19, Springer, pp.23-37, 2018, 978-3-319-75467-3. 10.1007/978-3-319-75468-0_3. hal-02539550 HAL Id: hal-02539550 https://hal.archives-ouvertes.fr/hal-02539550 Submitted on 15 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Surgical Anatomy of the insula Christophe Destrieux1, 2, Igor Lima Maldonado3, Louis-Marie Terrier1, 2, Ilyess Zemmoura1, 2 1 : Université François-Rabelais de Tours, Inserm, Imagerie et cerveau UMR 930, Tours, France 2 : CHRU de Tours, Service de Neurochirurgie , Tours, France 3 : Universidade Federal da Bahia, Laboratório de Anatomia e Dissecção, Departamento de Biomorfologia - Instituto de Ciências da Saúde, Salvador-Bahia, Brazil 1. Abstract The insula was for a long time considered as one of the most challenging areas of the brain. This is mainly related to its location, deep and medial to the fronto-parietal, temporal and fronto-orbital opercula. -

The Role of the Parietal Operculum
Haptic-based object directed behavior: the role of the parietal operculum. Student: Francesca Maule Advisor: Dott. Luigi Cattaneo A thesis submitted for the degree of Philosophiæ Doctor (PhD) Doctoral school in Cognitive and Brain Sciences XXVI cycle December 2013 II Abstract The aim of this thesis is to provide new insights about the role of the left human parietal operculum (OP) in sensory motor transformations in the context of object-directed behavior. This work is divided in two main parts: and introductive part about the theory underlying the sensory motor integration and the existing literature about the parietal operculum, and an experimental part in which the experiments realized during these three years are described. In Chapter 1, the theory underlying the sensory motor transformation in the visual modality and the possible functions of the different front- parietal circuits are described on the basis of the theory proposed in the literature. A specific paragraph is dedicated to ventral premotor cortex (PMv) and its role in visually guided grasping. The Chapter 2, is a review of the literature about the cytoarchitecture, the connectivity and the physiology of humans and non- human primates parietal operculum. In Chapter 3 the literature about the role of OP of primates and humans in sensory motor integration is reviewed together with some literature about studies on lesions. In the experimental part, four transcranial magnetic stimulation (TMS) experiments are described. The last two experiments have been grouped in a single major work and results have been discussed together. In Chapter 4 the Experiment I is described. This experiment aimed to characterize the fronto-parietal network involving the connection between left OP and ipsilateral primary motor cortex (M1). -

The Anterior Sylvian Point and the Suprasylvian Operculum
Neurosurg Focus 18 (6b):E2, 2005 The anterior sylvian point and the suprasylvian operculum GUILHERME CARVALHAL RIBAS, M.D., EDUARDO CARVALHAL RIBAS, AND CONSUELO JUNQUEIRA RODRIGUES, M.D. Clinical Anatomy Discipline of the Department of Surgery, University of São Paulo Medical School, Hospital Albert Einstein, São Paulo, Brazil Object. The sylvian fissure or lateral sulcus is the most identifiable feature of the superolateral brain surface and constitutes the main microneurosurgical corridor, given the high frequency of approachable intracranial lesions through this route. The anterior sylvian point (ASyP) divides this fissure in its main anterior and posterior rami and was evaluated in this study for its morphology, exact location, and sulcal and neural relationships to assess its suit- ability as an initial, visually identifiable landmark for further neuroimaging and intraoperative estimation of its adjoin- ing suprasylvian structures. Methods. This study is based on 32 formalin-fixed cerebral hemispheres. The brains were removed from the skulls of 16 cadavers after the introduction of plastic catheters through properly positioned burr holes; the number of speci- mens for some of the analyzed data differed because of incorrect positioning of catheters or damage to the studied structures caused by the initial steps of the study. The ASyP had a cisternal aspect in 94% of the specimens and was always located inferior to the triangular part of the inferior frontal gyrus, 2.3 Ϯ 0.5 cm in front of the inferior rolandic point. The ASyP was located underneath the 1.5-cm-diameter cranial area of the anterior aspect of the squamous suture. Its adjoining structures that compose the suprasylvian operculum have constant basic morphological configurations. -
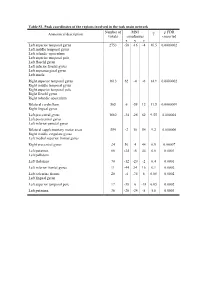
Table S1. Peak Coordinates of the Regions Involved in the Task Main
Table S1. Peak coordinates of the regions involved in the task main network Number of MNI p FDR Anatomical description T voxels coordinates corrected x y z Left superior temporal gyrus 2753 -58 -16 -4 16.5 0.0000002 Left middle temporal gyrus Left rolandic operculum Left superior temporal pole Left Heschl gyrus Left inferior frontal gyrus Left supramarginal gyrus Left insula Right superior temporal gyrus 1613 62 -4 -6 14.9 0.0000002 Right middle temporal gyrus Right superior temporal pole Right Heschl gyrus Right rolandic operculum Bilateral cerebellum 565 6 -58 12 11.5 0.0000009 Right lingual gyrus Left precentral gyrus 1682 -34 -26 62 9.55 0.000004 Left postcentral gyrus Left inferior parietal gyrus Bilateral supplementary motor areas 554 -2 10 54 9.2 0.000006 Right middle cingulate gyrus Left medial superior frontal gyrus Right precentral gyrus 24 56 4 44 6.9 0.00007 Left putamen 66 -24 -6 44 6.6 0.0001 Left pallidum Left thalamus 70 -12 -20 -2 6.4 0.0001 Left inferior frontal gyrus 11 -44 24 16 6.1 0.0002 Left calcarine fissure 20 -4 -74 8 6.08 0.0002 Left lingual gyrus Left superior temporal pole 17 -38 6 -18 6.05 0.0002 Left putamen 36 -28 -24 -8 5.8 0.0003 Table S2. Peak coordinates of the regions showing a main effect of item type Number of MNI p FDR Anatomical description T voxels coordinates corrected x y z Words – Pseudowords Left inferior frontal gyrus 96 -40 24 -6 7.6 0.000001 Left insula Left superior temporal pole Left middle temporal gyrus 141 -56 -36 -2 5.9 0.000015 Left precentral gyrus 26 -50 8 32 4.9 0.00025 Left inferior -
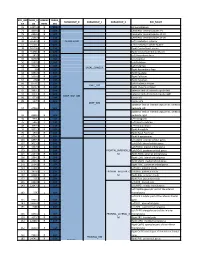
Core-Example1.Pdf
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital -

Variability of Human Cerebral Hemispheres Temporal
| archiv euromedica | 2019 | v ol. 9 | num . 1 | MOR ph O L O G Y , P H Y S I O L O G Y , 26 PAT H OLOGY VARIABILITY OF HUMAN CEREBRAL HEMISPHERES Article history: TEMPORAL OPERCULUM Received 20 February 2019 Received in revised form 25 March 2019 Accepted 29 March 2019 Yu.A. Gladilin1, O.A. Fomkina1, L.V. Muzurova1, E.A. Anisimova1, O.Yu. Aleshkina1, R.M. Khairullin2, O.V. Syrova1, V.V. Konnov3 1 Human anatomy department, Saratov State Medical University, Ministry of Healthcare of Russian Federation; Russia, 112, Bolshaya Kazachya Street, Saratov, 410012. E-mail: [email protected], tel: +7 917 208 6603 2 Department of Anatomy, Ulyanovsk State University, Russia , Ulyanovsk, L. Tolstoy Street, 42. E-mail: [email protected], tel: +7 917 607 3640 3 Department of Orthopedic Dentistry; Saratov State Medical University, Ministry of Healthcare of Russian Federation; Russia, 112, Bolshaya Kazachya Street, Saratov, 410012. E-mail: [email protected], tel: +7 917 026 5176 ABSTRACT — The temporal operculum is a region of the temporal lobe upper surface, which is bounded at the front by transverse temporal gyri, and from behind – by the end of the lateral cerebral sulcus. Aim of study: to identify the shape and the dimensional features of the temporal operculum in people aged 1–21. Fixed brain preparations (n = 47) were used to measure Fig. 1. Temporal operculum layout on the temporal lobe upper surface: the depth of the lateral sulcus at the level of the temporal operculum, and the temporal operculum length along its 1 — left temporal operculum, 2 — right temporal operculum outer edge; the temporal operculum area was calculated as well. -

Fractional Amplitude of Low-Frequency Fluctuation in Patients with Neovascular Glaucoma: a Resting-State Functional Magnetic Resonance Imaging Study
2150 Original Article Fractional amplitude of low-frequency fluctuation in patients with neovascular glaucoma: a resting-state functional magnetic resonance imaging study Yu-Qing Zhang#^, Meng-Ying Peng#, Shi-Nan Wu#, Chen-Yu Yu, Si-Yi Chen, Si-Wen Tan, Yi Shao^, Qiong Zhou^ Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang, China #These authors contributed equally to this work. Correspondence to: Yi Shao; Qiong Zhou. Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, No 17, YongWaiZheng Street, DongHu District, Nanchang 330006, China. Email: [email protected]; [email protected]. Background: Neovascular glaucoma (NVG) is a secondary refractory disease with a poor prognosis, and there are few advanced studies on its pathogenesis and treatment. In this research, the fractional amplitude of low-frequency fluctuation (fALFF) technology was used in resting-state functional magnetic resonance imaging (rsfMRI) to investigate intrinsic neuron activity in the patient’s brain with NVG. Methods: Sixteen patients with NVG (eight males and eight females) and 16 healthy controls (HCs) of similar age and sex were included. All patients and controls received rsfMRI scans, and the differences between the two groups in fALFF values were compared by independent sample t-test. Receiver operating characteristic (ROC) curves were used to compare fALFF values in the brain regions of NVG patients and HCs and assess accuracy. Finally, Pearson linear correlation analysis assessed the correlation between fALFF signals in brain regions and the clinical evaluation indicators of patients with NVG. Results: In patients with NVG, fALFF signal values in the right Rolandic operculum, left anterior cingulate and paracingulate gyri, and right caudate were significantly decreased. -

A Positron Emission Tomography Study of the Short-Term Maintenance of Verbal Information
The Journal of Neuroscience, January 15, 1996, 76(2):808-822 A Positron Emission Tomography Study of the Short-Term Maintenance of Verbal Information Julie A. Fiez,l13 Elizabeth A. Raife,’ David A. Balota,s Jacob P. Schwarql Marcus E. Raichle,1z2z3z4 and Steven E. Petersen1 ~3~4~5 1Departments of Neurology and Neurological Surgery, 2Anatomy and Neurobiology, 3Radiology, and 4The McDonnell Center for Higher Brain Function, Washington University School of Medicine, St. Louis, Missouri 63 I 10, and 5Department of Psychology, Washington University, St. Louis, Missouri 63 1 IO Positron emission tomography (PET) was used to investigate regions was specific to the maintenance condition. Conversely, the functional brain anatomy associated with the shot-t-term greater activation was found in a left middle insular region in the maintenance of linguistic information. Subjects were asked to silent counting than in the maintenance tasks. Based on con- retain five related words, unrelated words, or pseudowords verging results in this and previous studies, dorsolateral pre- silently for the duration of a 40 set PET scan. When brain frontal cortical areas appear to contribute to the maintenance of activity during these short-term maintenance tasks was com- both verbal and nonverbal information, whereas left frontal pared with a visual fixation control task, increases were found opercular regions appear to be involved specifically in the bilaterally in the dorsolateral prefrontal cortex and cerebellum, rehearsal of verbal material. Contrary to results found in other and medially in the supplementary motor area. Furthermore, studies of working memory, activation was not found in the effects of stimulus condition and recall performance were found inferior parietal cortex, suggesting that this area is involved in in the left frontal operculum. -

Evidence of Cortical Thickness Reduction and Disconnection in High
www.nature.com/scientificreports OPEN Evidence of cortical thickness reduction and disconnection in high myopia Ya‑Jun Wu1,3, Na Wu1,3, Xin Huang1, Jie Rao1, Li Yan1, Ling Shi1, Hui Huang1, Si‑Yu Li1, Fu‑Qing Zhou2* & Xiao‑Rong Wu1* High myopia (HM) is associated with impaired long‑distance vision. accumulating evidences reported that abnormal visual experience leads to dysfunction in brain activity in HM even corrected. However, whether the long‑term of abnormal visual experience lead to neuroanatomical changes remain unknown, the aim at this study is to investigate the alternation of cortical surface thickness in HM patients. 82 patients with HM (HM groups), 57 healthy controls (HC groups) were recruited. All participants underwent high‑resolution T1 and resting‑state functional magnetic resonance imaging (MRI) scans. The cortical thickness analysis was preformed to investigate the neuroanatomical changes in HM patients using computational anatomy toolbox (CAT 12) toolbox. Compare with HCs, HM patients showed decreased the cortical surface thickness in the left middle occipital gyrus (MOG), left inferior parietal lobule (IPL), right inferior temporal gyrus (ITG), right precuneus, right primary visual area 1 (V1), right superior temporal gyrus (STG), right superior parietal lobule (SPL), right occipital pole, and right the primary motor cortex (M1), and increased to the parietal operculum (OP4) (P < 0.01, FWE‑corrected), the mean cortical thickness of right orbitofrontal cortex (OFC), right dorsolateral prefrontal cortex (DLPFC) and right subcallosal cortex showed negatively correlation between clinical variables (axis length (ALM), the average macular thickness (AMT), keratometer (KER) 1, KER2, the mean KER, the mean macular fovea thickness (MFK), the refractive diopter) in HM patients. -

High Opiate Receptor Binding Potential in the Human Lateral Pain System
www.elsevier.com/locate/ynimg NeuroImage 30 (2006) 692 – 699 High opiate receptor binding potential in the human lateral pain system Ulf Baumga¨rtner,a,* Hans-Georg Buchholz,b Alexander Bellosevich,b Walter Magerl,a Thomas Siessmeier,b Roman Rolke,a Sabine Ho¨hnemann,c Markus Piel,c Frank Ro¨sch,c Hans-Ju¨rgen Wester,d Gjermund Henriksen,e Peter Stoeter,f Peter Bartenstein,b Rolf-Detlef Treede,a and Mathias Schreckenberger b aInstitute of Physiology and Pathophysiology, Johannes Gutenberg University, Saarstr. 21. D-55099 Mainz, Germany bDepartment of Nuclear Medicine, Johannes Gutenberg-University, Mainz, Germany cInstitute of Nuclear Chemistry, Johannes Gutenberg-University, Mainz, Germany dDepartment of Radiochemistry, Technical University, Munich, Germany eDepartment of Nuclear Medicine, Technical University, Munich, Germany fInstitute of Neuroradiology, Johannes Gutenberg-University, Mainz, Germany Received 5 July 2005; revised 14 October 2005; accepted 20 October 2005 Available online 7 December 2005 To determine how opiate receptor distribution is co-localized with the operculum (0.77). Factor analysis of interindividual variability of distribution of nociceptive areas in the human brain, eleven male opiate receptor BP revealed four factors (95% explained variance): (1) healthy volunteers underwent one PET scan with the subtype- operculo-insular areas, ACC, MCC and putamen, (2) amygdala and nonselective opioidergic radioligand [18F]fluoroethyl-diprenorphine thalamus, (3) caudate and thalamus, (4) SI/MI and MCC. under resting conditions. The binding potential (BP), a parameter for Nociceptive areas of the lateral pain system (frontoparietal the regional cerebral opioid receptor availability, was computed using operculum and insula) have opiate receptor BPs significantly higher the occipital cortex as reference region.