Lithium Concentrations in Brine Springs Near Lake Winnipegosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Keymap
Nabel Nueltin Lake Lake COLVIN LAKE NUELTIN LAKE CARIBOU RIVER PROVINCIAL PARK Nejanilini Shannon PARK RESERVE HUDSON Lake PROVINCIAL PARK Lake Cochrane R North Seal River Seal Churchill Nicklin River L Shethanei BAY Lake Lac Brochet Tadoule Lake Whiskey Jack Lake River North WAPUSK Knife Seal Lake Churchill River NATIONAL NUMAYKOOS SAND LAKESSouth PROVINCIAL P ARK PROVINCIAL PARK PARK Big Reindeer Sand Lake Lake Northern Southern Indian Lake Thorsteinson Churchill Lake Fidler River Indian Lake Port Nelson Vandekerckhove Goldsand Gauer Barrington Lake Lake Lake Lake Lake River Opachuanau Lake AMISK PARK RESERVE Waskaiowaka River River River Baldock Lake Stephens Lake Lake Rat Gods Leaf Rapids Granville Nelson Russell Lake Split Lake River Rat Mynarski Lake Lakes Lake Hayes Highrock Lake Churchill THOMPSON PAINT LAKE PROVINCIAL PARK EAST PAINT LAKE PARK RESERVE River Sipiwesk Knee Lake River Lake Oxford River Edmund Snow Lake Lake Lake Cross Gods Wekusko Grass FLIN FLON Lake Walker Grass Sucker Lake Lake GRASS RIVER PROVINCIAL PARK Lake Sharpe Lake Red Red Sucker Lake Molson Lake R North Moose CLEARWATER Lake LAKE Nelson PROVINCIAL PARK Island Playgreen L Lake THE PAS LITTLE LIMESTONE LAKE PROVINCIAL PARK Cedar WALTER COOK UPLANDS CAVES ECOLOGICAL LAKE RESERVE TOWN OF Lake GRAND RAPIDS WINNIPEG L A C L W A K I E N W N I I GRAND ISLAND N P RED DEER N PARK RESERVE E I SOUTHERN MANITOBA MUNICIPAL P G GOOSE ISLANDS PEMICAN ISLAND E O PARK RESERVE PARK RESERVE G S O I MOUNTAIN S S (NORTH) I S Swan KEY MAP BIRCH ISLAND R Lake CHITEK LAKE PARK RESERVE PROVINCIAL PARK SWAN Berens LAKE Bowsman MINITONAS- River DUCK Fishing SWAN BOWSMAN BAY VALLEY Swan River L Minitonas WESTSwan KINWOW BAY PARK RESERVE LAKE Benito WATERHEN ST MARTIN MOUNTAIN STURGEON BAY (SOUTH) HOMEBROOK- PARK RESERVE ATIKAKI PEONAN POINT GRAHAMDALE FISHER BAY DUCK MOUNTAIN Lake PARK PROVINCIAL PARK Winnipegosis PROVINCIAL RESERVE LAKE ETHELBERT St. -

Faculty of Graduate Studies
3'A.C|IJL:IY 0F t'RA:)LlrÊ'TE Ë'TJIÏE'1 DEP/ìRTMfiÌT OF,- T],VJ ]. EI$GTIVEEBSNI] tilIÏVElRlrllty 03' j4AN:]"T0BA AN ÍN\TfißTTI.íTITTOJ\T O}' T}IE ÐTVERåJ.OIf C}:.rÎ NOR'IIìTB.N }4ANTTOEA I{ATE¡I.:3 J,NTO ].,AKE }IIAJü OÉA l- Jt t. .4. Fl:-¡40I{ n TI]Tfl:I:ì J:I{,PÅR]TÏAL FLI"LF],ffÆI\T 'sUBT\MTTEÐ CIF T'TIE REQLÍSAEME}Í?i3 iIÜR TTIE NEGBEE ÐF j/ÛslER Olr SÜ3,Ï:IùGE APRTT L?6"r acitro\'fT.m)Gm{msrs The ç'råter v¡Èshes to e:qpress hi s mrnest appreciatf on to Jan:ice, A.LLísou and. Davåd., Írz acknowledgenaent of thefr patÍenee anð u.nåerstaadågg ín vi.ll-iragly glvfrug 'ap thefr ri.ghts to 'the eompanS"orashåp anå at'Lentínn r¡f a husba.trð and' fether during the many hours sPent on this thesis. I&ny that:les ave alnc¡ åt¿Ë to Professor E" Ktliper for his årrspåratiou in the i,nitåatlon of, this stud-y and' his guldance ancl assåstanee tÏ.lz"oughout " The w.riter would also Like to eryrers his appreciation to the månage¡geni and ,sf"aff af Underwooð, Mçtellan and. AssocÍates, for their ånteres'L a.rio Ínvolvement ån thj"s thesis, especially to Meser"s" A. G. Spaffor"d" and R. c. La'tta, vå'bhout whose gtrÍdance and assÍstance this thesís j..n j"ts fånãt fow¡r wou[d. t ot be possfble' The r+¡"íter also wÍshes to acknovf-ed-ge grateful-Ly the assfs.tance of 3,fr. -

Pdfs GST-HST Municipal Rebates 2019 E Not Finished.Xlsx
GST/HST Incremental Federal Rebate for Municipalities Report - January 1 to December 31, 2019 Manitoba PAYMENT LEGAL NAME CITY NAME FSA AMOUNT 2625360 MANITOBA ASSOCIATION INC. NEEPAWA R0J $2,993.73 285 PEMBINA INC WINNIPEG R2K $10,624.47 4508841 MANITOBA ASSOCIATION INC WINNIPEG R2K $517.02 474 HARGRAVE CORPORATION WINNIPEG R3A $2,504.76 6869166 MANITOBA LTD. SANFORD R0G $7,370.38 ACADEMY ROAD BUSINESS IMPROVMENT ZONE WINNIPEG R3N $1,389.15 AGASSIZ WEED CONTROL DISTRICT BEAUSEJOUR R0E $549.30 ALTONA RURAL WATER SERVICES CO-OP LTD ALTONA R0G $1,860.62 ARBORG BI-FROST PARKS & RECREATION COMMISSION ARBORG R0C $5,326.89 ARGYLE-LORNE-SOMERSET WEED CONTROL DISTRICT BALDUR R0K $553.10 ARLINGTONHAUS INC. WINNIPEG R2K $11,254.49 ARTEMIS HOUSING CO-OP LTD WINNIPEG R3A $2,784.09 ASTRA NON-PROFIT HOUSING CORPORATION WINNIPEG R2K $2,993.66 AUTUMN HOUSE INC. WINNIPEG R3E $3,532.89 B&G UTILITIES LTD BRANDON R7B $3,643.38 BAPTIST MISSION APARTMENTS INC. WINNIPEG R3E $2,224.34 BARROWS COMMUNITY COUNCIL BARROWS R0L $3,837.41 BEAUSEJOUR BROKENHEAD DEVELOPMENT CORP BEAUSEJOUR R0E $3,583.19 BETHANIAHAUS INC. WINNIPEG R2K $17,881.45 BIBLIOTHÉQUE MONTCALM LIBRARY SAINT-JEAN-BAPTISTE R0G $180.01 BIBLIOTHÉQUE REGIONALE JOLYS REGIONAL LIBRARY SAINT-PIERRE-JOLYS R0A $267.88 BIBLIOTHÉQUE TACHÉ LIBRARY LORETTE R0A $851.71 BISSETT COMMUNITY COUNCIL BISSETT R0E $2,919.53 BLUMENFELD HOCHFELD WATER CO-OP LTD WINKLER R6W $770.13 BLUMENORT SENIOR CITIZENS HOUSING INC. STEINBACH R5G $515.67 BOISSEVAIN - MORTON LIBRARY AND ARCHVIES BOISSEVAIN R0K $784.80 BOISSEVAIN AND MORTON -

The Community Living Funding Crisis in Westman and Parkland a REPORT on 15 AGENCIES
The Community Living Funding Crisis in Westman and Parkland A REPORT ON 15 AGENCIES An analysis of systemic problems and recommendations to address these concerns April 2014 Dr. Megan McKenzie, Conflict Specialist Table of Contents Contents Executive Summary __________________________________________________________ 1 Summary of Recommendations _________________________________________________ 3 The Funding Crisis ___________________________________________________________ 6 ACL Swan River ____________________________________________________________ 31 ACL Virden ________________________________________________________________ 33 Brandon Community Options __________________________________________________ 36 Community Respite Services (Brandon) __________________________________________ 39 COR Enterprises Inc. (Brandon) ________________________________________________ 42 Frontier Trading Company Inc. (Minnedosa) ______________________________________ 45 Grandview Gateways Inc. _____________________________________________________ 47 Parkland Residential and Vocational Services Inc. (Dauphin) _________________________ 51 Prairie Partners (Boissevain) __________________________________________________ 54 ROSE Inc. (Ste. Rose du Lac) _________________________________________________ 56 Rolling Dale Enterprises Inc.(Rivers) ____________________________________________ 59 Southwest Community Options (Ninette) _________________________________________ 61 Touchwood Park (Neepawa) ___________________________________________________ 65 Westman -
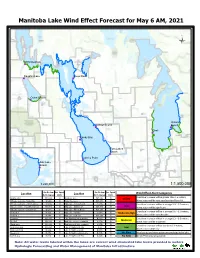
Manitoba Lake Wind Effect Forecast for May 6 AM, 2021 Ü
Manitoba Lake Wind Effect Forecast for May 6 AM, 2021 Ü Winnipegosis !P Dauphin Lake Steep Rock !P !P Ochre Beach !P Victoria Lundar Beach Beach !P !P Gimli !P Sandy Bay !P Twin Lakes Beach !P Lynchs Point !P Oak Lake Beach !P 1:220,000 1:1,500,000 Ave.Dir.from Ave. Speed Ave.Dir.from Ave. Speed Wind Effect Alert Categories Location North (degree) (km/h) Location North (degree) (km/h) Crest level increase will be greater than 1.5 meters; Dauphin Lake NW (320) 21 Manipogo area NW (336) 15 Severe MB Lake west side - Sandy Bay W (292) 18 Crane River area NW (335) 12 Wave action will be very significant and forceful. MB Lake south side - Twin Beaches W (274) 17 Wpg Lake - Victoria Beach W (289) 12 Crest level increase will be in a range 0.9 ~ 1.5 meters; High MB Lake middle - Vogar/The Narrows NW (311) 12 Wpg Lake - Gimli Beach NW (318) 13 Wave action will be significant. MB Lake east side - Lundar Beach W (281) 12 Wpg Lake - Alberta Beach NW (293) 11 Crest level increase will be in a range 0.6 ~ 0.9 meters; Northend 1 N (351) 9 Wpg Lake - O'Hanly W (292) 11 Moderate-High Northend 2 N (344) 11 Wpg Lake south side NW (294) 11 Wave action will be considerable. Guynemer NW (337) 12 Lake Winnipegosis south side N (343) 19 Crest level increase will be in a range 0.3 ~ 0.6 meters; Moderate The Bluff NW (331) 14 Oak Lake east side community W (276) 18 Wave action will be moderate. -

Municipal Officials Directory 2021
MANITOBA MUNICIPAL RELATIONS Municipal Officials Directory 21 Last updated: September 23, 2021 Email updates: [email protected] MINISTER OF MUNICIPAL RELATIONS Room 317 Legislative Building Winnipeg, Manitoba CANADA R3C 0V8 ,DPSOHDVHGWRSUHVHQWWKHXSGDWHGRQOLQHGRZQORDGDEOH0XQLFLSDO2IILFLDOV'LUHFWRU\7KLV IRUPDWSURYLGHVDOOXVHUVZLWKFRQWLQXDOO\XSGDWHGDFFXUDWHDQGUHOLDEOHLQIRUPDWLRQ$FRS\ FDQEHGRZQORDGHGIURPWKH3URYLQFH¶VZHEVLWHDWWKHIROORZLQJDGGUHVV KWWSZZZJRYPEFDLDFRQWDFWXVSXEVPRGSGI 7KH0XQLFLSDO2IILFLDOV'LUHFWRU\FRQWDLQVFRPSUHKHQVLYHFRQWDFWLQIRUPDWLRQIRUDOORI 0DQLWRED¶VPXQLFLSDOLWLHV,WSURYLGHVQDPHVRIDOOFRXQFLOPHPEHUVDQGFKLHI DGPLQLVWUDWLYHRIILFHUVWKHVFKHGXOHRIUHJXODUFRXQFLOPHHWLQJVDQGSRSXODWLRQV,WDOVR SURYLGHVWKHQDPHVDQGFRQWDFWLQIRUPDWLRQRIPXQLFLSDORUJDQL]DWLRQV0DQLWRED([HFXWLYH &RXQFLO0HPEHUVDQG0HPEHUVRIWKH/HJLVODWLYH$VVHPEO\RIILFLDOVRI0DQLWRED0XQLFLSDO 5HODWLRQVDQGRWKHUNH\SURYLQFLDOGHSDUWPHQWV ,HQFRXUDJH\RXWRFRQWDFWSURYLQFLDORIILFLDOVLI\RXKDYHDQ\TXHVWLRQVRUUHTXLUH LQIRUPDWLRQDERXWSURYLQFLDOSURJUDPVDQGVHUYLFHV ,ORRNIRUZDUGWRZRUNLQJLQSDUWQHUVKLSZLWKDOOPXQLFLSDOFRXQFLOVDQGPXQLFLSDO RUJDQL]DWLRQVDVZHZRUNWRJHWKHUWREXLOGVWURQJYLEUDQWDQGSURVSHURXVFRPPXQLWLHV DFURVV0DQLWRED +RQRXUDEOHDerek Johnson 0LQLVWHU TABLE OF CONTENTS MANITOBA EXECUTIVE COUNCIL IN ORDER OF PRECEDENCE ............................. 2 PROVINCE OF MANITOBA – DEPUTY MINISTERS ..................................................... 5 MEMBERS OF THE LEGISLATIVE ASSEMBLY ............................................................ 7 MUNICIPAL RELATIONS .............................................................................................. -

CHARACTERISTICS and DISTRIBUTION of LAKES 631 Lake Winnipegosis, Lying to the North of Lake Manitoba
CHARACTERISTICS AND DISTRIBUTION OF LAKES 631 Lake Winnipegosis, lying to the north of Lake Manitoba. at an elevation of 828 feet above sea-level, is about 130 miles long, by 20 miles in maximum breadth. It covers an area of 2080 square miles, and has a maximum depth of 38 feet. It is fed by many small streams from the west, and by the overflow of Lake Dauphin (840 feet above sea-level) through Mossy River. The outlet is by the very indirect way of Waterhen River, through Waterhen Lake, to Lake Manitoba. The total area of the lakes in the Winnipeg basin is 13,500 square miles. Lake Wollaston, 1300 feet above sea-level, is the ultimate source River of the Reindeer River, one of the chief tributaries of the Churchill Churchill. River. It is about 800 square miles in area, and discharges by two outlets—to the north by the Stone River into the extreme eastern arm of Lake Athabasca, and to the south-east by the Cochrane River into Reindeer Lake. Reindeer Lake, 1150 feet above sea-level, is 135 miles long, and has an area of 2490 square miles; the Reindeer River carries its overflow to the Churchill River. The rivers in Labrador are often like strings of lakes, and divide River Rupert. and unite again in their course, while the lakes frequently discharge in two directions. Lake Mistassini, 1350 feet above sea-level, the largest lake, is practically two parallel lakes divided by a range of islands in the centre. The western lake is 90 miles long by 13 to 17 miles wide, and the eastern lake is 60 miles long by 5 to 10 miles wide, the greatest depths being 300 to 400 feet. -

Pdfs GST-HST Municipal Rebates 2018 E.Xlsx
GST/HST Incremental Federal Rebate for Municipalities Report - January 1 to December 31, 2018 Manitoba PAYMENT LEGAL NAME CITY NAME FSA AMOUNT 2625360 MANITOBA ASSOCIATION INC. NEEPAWA R0J $1,112.09 285 PEMBINA INC WINNIPEG R2K $11,916.31 4508841 MANITOBA ASSOCIATION INC WINNIPEG R2K $1,519.18 474 HARGRAVE CORPORATION WINNIPEG R3A $2,653.46 6869166 MANITOBA LTD. SANFORD R0G $6,712.69 7049651 MANITOBA ASSOCIATION INC. WINNIPEG R3B $330,332.94 ACADEMY ROAD BUSINESS IMPROVMENT ZONE WINNIPEG R3N $6,535.53 AGASSIZ WEED CONTROL DISTRICT BEAUSEJOUR R0E $244.81 ALTONA RURAL WATER SERVICES CO-OP LTD ALTONA R0G $2,145.25 ARBORG BI-FROST PARKS & RECREATION COMMISSION ARBORG R0C $4,415.00 ARGYLE-LORNE-SOMERSET WEED CONTROL DISTRICT BALDUR R0K $322.95 ARLINGTONHAUS INC. WINNIPEG R2K $6,353.08 ARTEMIS HOUSING CO-OP LTD WINNIPEG R3A $1,573.30 ASTRA NON-PROFIT HOUSING CORPORATION WINNIPEG R2K $2,548.64 AUTUMN HOUSE INC. WINNIPEG R3E $7,366.31 BAPTIST MISSION APARTMENTS INC. WINNIPEG R3E $3,828.08 BARROWS COMMUNITY COUNCIL BARROWS R0L $2,713.78 BEAUSEJOUR BROKENHEAD DEVELOPMENT CORP BEAUSEJOUR R0E $4,669.11 BETELSTADUR HOUSING COOPERATIVE LTD WINNIPEG R3A $669.10 BETHANIAHAUS INC. WINNIPEG R2K $6,160.12 BIBLIOTHÉQUE MONTCALM LIBRARY SAINT-JEAN-BAPTISTE R0G $177.01 BIBLIOTHÉQUE REGIONALE JOLYS REGIONAL LIBRARY SAINT-PIERRE-JOLYS R0A $761.21 BIBLIOTHÉQUE TACHÉ LIBRARY LORETTE R0A $729.46 BISSETT COMMUNITY COUNCIL BISSETT R0E $4,346.40 BLUMENFELD HOCHFELD WATER CO-OP LTD WINKLER R6W $540.72 BLUMENORT SENIOR CITIZENS HOUSING INC. STEINBACH R5G $834.10 BOISSEVAIN -

Public Accounts of the Province of Manitoba
HIL-GOV L14EF33 R4 2 6 1980/81 public v. 2 accounts 1980-81 volume 2-supplementary information UNIVERS TY OF NEW BRUNSWICK LIBRAR ES 3 9950 27 0930 MANITOBA for the year ended March 31, 1981 public accounts 1980-81 volume 2-supplementary information MANIT1BA for the year ended March 31, 1981 0 4 3 3 3 Digitized by the Internet Archive in 2019 with funding from University of Alberta Libraries https://archive.org/details/publicaccountsof02prov_0 PUBLIC ACCOUNTS 1980-81 GOVERNMENT OF THE PROVINCE OF MANITOBA TABLE OF CONTENTS Salaries and Wages Paid to Employees, for the fiscal year ended 31st March, 1981 . 7 Cash Payments to Corporations, Government Agencies, Firms, Individuals, Municipalities, Cities, Towns and Villages, for the fiscal year ended 31st March, 1981 .77 GOVERNMENT OF THE PROVINCE OF MANITOBA lllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllll CASH PAYMENTS TO CORPORATIONS, FIRMS, GOVERNMENT AGENCIES, INDIVIDUALS, MUNICIPALITIES, CITIES, TOWNS AND VILLAGES BY THE GOVERNMENT OF THE PROVINCE OF MANITOBA Cash paid to corporations, firms, government agencies, individuals, municipalities, cities, towns and villages, arranged in alphabetical order to show the disbursements to each payee where the total payments exceed $2,000.00 for the year ended March 31,1981. Payments are presented on a gross basis to show all amounts disbursed for purchase of goods and services, investment and loan transactions, grant payments, refunds and amounts held in trust on behalf of others. Payments made to United States suppliers are presented in United States dollars. CASH PAYMENTS TO CORPORATIONS, ETC., 1980-81 77 GOVERNMENT OF THE PROVINCE OF MANITOBA “A” Abigosis, Norbert, Camperville . 2,750.00 A. -

RIVERS and LAKES 11 the Great Lakes.—Table 4 Shows the Length
RIVERS AND LAKES 11 The Great Lakes.—Table 4 shows the length, breadth, area, elevation above sea-level and maximum depth of each of the Great Lakes. i.—Area, Elevation and Depth of the Great Lakes. Maximum Elevation Lakes. Length. Breadth. depth. Area. above sea-level. square miles. miles. feet. miles. feet. Superior... 383 160 1,180 31,810 602-29 Michigan.. 320 118 870 22,400 581-13 Huron 247 101 750 23,010 581-13 St. Clair.., 26 24 23 460 575-62 Erie 241 57 210 9,940 572-52 Ontario 180 53 738 7,540 246-17 Lake Superior, with its area of 31,810 square miles, is the largest body of fresh water in the world. As the international boundary between Canada and the United States passes through the centre of lakes Superior, Huron, Erie, St. Clair and Ontario, only half of the areas of these lakes given in the above statement is Canadian. The whole of lake Michigan is within United States territory. From the western end of lake Superior to the mouth of the St. Lawrence there is, with the aid of the canal system, a continuous navigable waterway. The total length of the St. Law rence river from the head of the St. Louis river to Pointe-des-Monts, at the entrance of the gulf of St. Lawrence, is 1,900 miles. The tributaries of the St. Lawrence, several of which have themselves important tributaries, include the Ottawa river, 685 miles long, the St. Maurice river, 325 miles long, and the Sague- nay (to head of Peribonka), 405 miles long. -

05260 Sask Watershed Report.Qxd:State of The
Saskatchewan Watershed Authority STATE OF THE WATERSHED REPORTING FRAMEWORK Monitoring and Assessment Branch Stewardship Division January 2006 Suite 420-2365 Albert Street Regina, Saskatchewan S4P 4K1 www.swa.ca Cover photos courtesy of (top-bottom): Saskatoon Tourism; Nature Saskatchewan; Saskatchewan Watershed Authority; Saskatchewan Watershed Authority; Wawryk Associates Ltd.; Ducks Unlimited Canada; Saskatchewan Watershed Authority; Nature Saskatchewan; and Saskatchewan Industry and Resources. Minister’s Message From the grasslands in the southwest to the Taiga Shield lakes of the north, we in Saskatchewan are blessed with richly diverse ecosystems. Over the past few decades, we have come to understand more about the nature of these ecosystems, and how we rely on them to support our economy and our overall quality of life. We have also increased our understanding of the responsibility we bear to assess, protect and improve our province’s environmental health, in balance with our social and economic priorities, for the good of present and future generations. We face a number of challenges regarding our water’s quality and availability. It is up to Saskatchewan people to determine how we will meet these challenges. The Saskatchewan government has taken a leadership role in assessing, protecting and managing our source waters by addressing them at the watershed level. The State of the Watershed Reporting Framework represents a critical step in understanding our relationship with Saskatchewan’s watersheds, as well as our role as stewards of our environment. For the first time in Saskatchewan, this framework will integrate the information collected by numerous provincial and federal agencies and present it in an easily understandable, technically sound report- card format. -

Supplementary Map 2: MMF Night Hunting and Night Lighting Agro-Zone Boundary with Rural Municipalities (R.M.)
Supplementary Map 2: MMF Night Hunting and Night Lighting Lake Agro-Zone Boundary with Lake Winnipeg Winnipegosis Rural Municipalities (R.M.) Legend Mountain Agro-Zone Boundary Agro-Manitoba Non-Agro Manitoba Riding Mountain National Park Waterbody Minitonas Rural Municipality (R.M.) Swan Manitoba Provincial Boundary River 0 5 10 20 30 40 Kilometres Miles Mountain 0 5 10 20 30 40 Data Source: MLI, Geogratis Scale: 1:1,150,000 Projection: UTM Zone 14 NAD 83 (when printed 11" x 17") ¯ Date: April 3, 2019 Mossey River Ethelbert SS KK Shell River Lawrence Grahamdale OO NN Hillsburg Dauphin Dauphin Gilbert Lake Grandview Plains Siglunes Fisher Shellmouth-Boulton Ochre Ste. Bifrost Rose River Alonsa Eriksdale Russell Silver McCreary Creek Rossburn Armstrong Park Coldwell Gimli Glenella Birtle Clanwilliam Alexander Lake Ellice Shoal DISCLAIMER: This m ap is a ge n e ralize d re pre se n tatioLn aokfe the ManSittorabath Mclae tiirs Fe dHe raartiroisno’sn (the “MMF”) Agro-Zon e Bou n dary for the pu rpose s of Night Hu n tin g Manitoba St. Rosedale an d Night Lightin g by Man itoba Me tis Harve ste rs an d Citize n s an d for n o othe r pu rpose . This m ap is for illu strative an d re fe re n ce pu rpose s on ly. It m u st be u se d in Laurent addition to the m ate rials available at: http://w w w .m m f.m b.ca, w hich m ay be u pdate d from tim e to tim e .