Micro Motion Corrosion Guide User Guide Before You Begin GI-00415 October 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
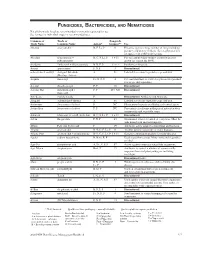
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Investigation of Condensed and Early Stage Gas Phase Hypergolic Reactions Jacob Daniel Dennis Purdue University
Purdue University Purdue e-Pubs Open Access Dissertations Theses and Dissertations Fall 2014 Investigation of condensed and early stage gas phase hypergolic reactions Jacob Daniel Dennis Purdue University Follow this and additional works at: https://docs.lib.purdue.edu/open_access_dissertations Part of the Propulsion and Power Commons Recommended Citation Dennis, Jacob Daniel, "Investigation of condensed and early stage gas phase hypergolic reactions" (2014). Open Access Dissertations. 256. https://docs.lib.purdue.edu/open_access_dissertations/256 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. i INVESTIGATION OF CONDENSED AND EARLY STAGE GAS PHASE HYPERGOLIC REACTIONS A Dissertation Submitted to the Faculty of Purdue University by Jacob Daniel Dennis In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2014 Purdue University West Lafayette, Indiana ii To my parents, Jay and Susan Dennis, who have always pushed me to be the person they know I am capable of being. Also to my wife, Claresta Dennis, who not only tolerated me but suffered along with me throughout graduate school. I love you and am so proud of you! iii ACKNOWLEDGEMENTS I would like to express my sincere gratitude to my advisor, Dr. Timothée Pourpoint, for guiding me over the past four years and helping me become the researcher that I am today. In addition I would like to thank the rest of my PhD Committee for the insight and guidance. I would also like to acknowledge the help provided by my fellow graduate students who spent time with me in the lab: Travis Kubal, Yair Solomon, Robb Janesheski, Jordan Forness, Jonathan Chrzanowski, Jared Willits, and Jason Gabl. -

US EPA, Pesticide Product Label, LIME-SULFUR SOLUTION
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, DC 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION January 29, 2018 Michael Kellogg Regulatory Consultant c/o Pyxis Regulatory Consulting, Inc. 4110 136th St. Ct NW Gig Harbor, WA 98332 Subject: Notification per PRN 98-10 – New Primary Brand Name. Product Name: Lime-Sulfur Solution Agricultural Fungicide EPA Registration Number: 61842-30 Application Date: 12/21/2017 Decision Number: 537150 Dear Michael Kellogg: The Agency is in receipt of your Application for Pesticide Notification under Pesticide Registration Notice (PRN) 98-10 for the above referenced product. The Registration Division (RD) has conducted a review of this request for its applicability under PRN 98-10 and finds that the action requested falls within the scope of PRN 98-10. The label submitted with the application has been stamped “Notification” and will be placed in our records. Should you wish to add/retain a reference to the company’s website on your label, then please be aware that the website becomes labeling under the Federal Insecticide Fungicide and Rodenticide Act and is subject to review by the Agency. If the website is false or misleading, the product would be misbranded and unlawful to sell or distribute under FIFRA section 12(a)(1)(E). 40 CFR 156.10(a)(5) list examples of statements EPA may consider false or misleading. In addition, regardless of whether a website is referenced on your product’s label, claims made on the website may not substantially differ from those claims approved through the registration process. Therefore, should the Agency find or if it is brought to our attention that a website contains false or misleading statements or claims substantially differing from the EPA approved registration, the website will be referred to the EPA’s Office of Enforcement and Compliance. -

Limestone Resources of Western Washington
State of Washington DANIEL J. EVANS, Governor Department of Conservation H. MA URI CE AHLQUIST, Director DIVISION OF MINES AND GEOLOGY MARSHALL T. HUNTTING, Supervisor Bulletin No. 52 LIMESTONE RESOURCES OF WESTERN WASHINGTON By WILBERT R. DANNER With a section on the UME MOUNTAIN DEPOSIT By GERALD W. THORSEN STATII PRINTING PLANT, OLYMPI A, WASH, 1966 For sale by Department of Conservation, Olympia, Washington. Price, $4,50 FOREWORD Since the early days of Washington's statehood, limestone has been recognized as one of the important mineral resources _of the State. The second annual report of the Washington Geological Survey, published in 1903, gave details on the State's limestone deposits, and in later years five other reports published by the Survey and its successor agencies hove given additional information on this resource. Still other reports by Federal and private agencies hove been published in response to demands for data on limestone here. Although some of the earlier reports included analyses to show the purity of the rocks, very few of the samples for analysis were taken systemati cally in a way that would fairly represent the deposits sampled. Prior to 1900 limestone was produced for use as building stone here, and another important use was for the production of burned Ii me . Portland cement plants soon became leading consumers of Ii mestone, and they con tinue as such to the present time . Limestone is used in large quantities in the pulp industry in the Northwest, and in 1966 there was one commercial lime-burning plant in the State. Recognizing the potential for industrial development in Washington based on more intensive use of our mineral resources, and recognizing the need to up-dote the State's knowledge of raw material resources in order to channel those resources into the State's growing economy, the Industrial Row Materials Advisory Committee of the Deportment of Commerce and Economic Development in 1958 recommended that a comprehensive survey be made of the limestone resources of Washington. -

Environmental Protection Agency § 712.30
Environmental Protection Agency § 712.30 (2) A confidentiality statement § 712.30 Chemical lists and reporting signed and dated by an authorized per- periods. son located at the plant site or cor- (a)(1) Persons subject to this subpart porate headquarters of the respondent B must submit a Preliminary Assess- company. ment Information Manufacturer’s Re- (3) The specific chemical name and port for each chemical substance or Chemical Abstracts Service (CAS) Reg- mixture that is listed or designated in istry Number listed in 40 CFR 712.30. this section. (4) The name, company, address, city, State, ZIP code, and telephone number (2) Unless a respondent has already of a person who is submitting the form, prepared a Manufacturer’s Report in which may be a person located at a conformity with conditions set forth in plant site or corporate headquarters paragraph (a)(3) of this section, the in- that will serve as the respondent, and formation in each Manufacturer’s Re- will be able to answer questions about port must cover the respondent’s latest the information submitted by the com- complete corporate fiscal year as of the pany to EPA. A respondent to this sub- effective date. The effective date will part must include the appropriate Dun be 30 days after the FEDERAL REGISTER and Bradstreet Number for each plant publishes a rule amendment making site reported. the substance or mixture subject to (5) The plant site activities, such as this subpart B. the manufacturing of a chemical sub- (3) Persons subject to this subpart B stance, including the total quantity of need not comply with the requirements the chemical substance (in kilograms) of paragraph (a)(2) of this section if imported in bulk during the reporting they meet either one of the following period. -

Crude Ticl4 Purification: a Review of the Current State of the Art and Future Opportunities
Crude TiCl4 purification: a review of the current state of the art and future opportunities L. Hockaday and A. Kale Mintek, Randburg, South Africa Raw titanium tetrachloride (TiCl4) is produced by chlorination of titania feedstock. Prior to its utilization in the manufacture of titanium dioxide pigment or titanium metal, the TiCl4 is purified by adding reagents that react with the impurities in the crude TiCl4, followed by distillation of the mixture. The current state of the art in purification of crude TiCl4 was surveyed. Tests were conducted at the laboratory scale to assess the efficiency of various additives used in the purification process to minimize the vanadium content in the distillate. The effects of various reagents, namely oleic acid, sodium oleate, potassium oleate, and copper on the purification of crude TiCl4 were compared. The purified TiCl4 was water-clear in aspect and analysed < 2 ppm vanadium for all reagents tested. Possible reactions between the impurities in the crude TiCl4 and the reagents are investigated thermodynamically with the FactSage program. INTRODUCTION Titanium tetrachloride (TiCl4) is obtained by the chlorination of a titanium-bearing mineral such as ilmenite and rutile, as well as from titanium slag. The chlorination process usually takes place in a fluidized bed in the presence of chlorine gas and a reducing agent such as petroleum coke, resulting in crude titanium tetrachloride. TiCl4 is an intermediate product in the production of titanium dioxide (TiO2) pigment and titanium metal. The pure form of titanium dioxide is used in the pigment industry for paints, food colouring etc., and titanium metal is widely used in the aerospace, medicine, sport, and semiconductor production industries. -

United States Patent Office 2,978,864
2,978,864 United States Patent Office Patented Apr. 11, 1961 2 use of previously known explosives containing ammonium nitrate by reason of the fact that they can be easily and 2,978,864 simply prepared at the site at which they are to be used. In many instances, the explosives can be prepared di AMMONUMNTRATE EXPLOSIVES rectly in the bore hole by simply placing the ammonium Leonard A. Stengel, Terre Haute, Ind., assignor to Com nitrate charge in the bore hole and passing a suitable mercial Solvents Corporation, Terre Haute, Ind., a amount of gaseous monomethylamine through the am-- corporation of Maryland - monum nitrate therein. - - My new explosive compositions can also be prepared No Drawing. Filed May 19, 1958, ser, No. 735,987 O in the bore hole by dissolving the monomethylamine and 7 Claims. (CI. 60-35.4) a liquid in which it is soluble, such as ethanol, and pouring the resulting amine solution over the ammonium nitrate charge. The charge can, of course, include car bon black, fuel oil, or other ingredients ordinarily incor My invention relates to new ammonium nitrate com 5 porated into ammonium nitrate explosive compositions. positions, and more particularly to compositions pre The ammonium nitrate-monomethylamine compositions pared from ammonium nitrate and monomethylamine of my invention require primer charges to initiate and which are suitable for use as explosives and propellants propagate detonation waves through the charge. If the and to processes for using same. bore hole in which the exjplosive is to be detonated is Ammonium nitrate has long been known to be flam 20 wet, horizontal, or slanting, the ammonium nitrate may mable and explosive and these properties have been made be placed in a container such as a polyethylene. -

Continuous Flow Nitration in Miniaturized Devices
Continuous flow nitration in miniaturized devices Amol A. Kulkarni Review Open Access Address: Beilstein J. Org. Chem. 2014, 10, 405–424. Chem. Eng. & Proc. Dev. Division, CSIR-National Chemical doi:10.3762/bjoc.10.38 Laboratory, Pune – 411 008, India, phone: +91-20-25902153 Received: 08 August 2013 Email: Accepted: 14 January 2014 Amol A. Kulkarni - [email protected] Published: 14 February 2014 This article is part of the Thematic Series "Chemistry in flow systems III". Keywords: continuous flow; flow chemistry; nitration; nitric acid; microreactors; Guest Editor: A. Kirschning tubular reactor © 2014 Kulkarni; licensee Beilstein-Institut. License and terms: see end of document. Abstract This review highlights the state of the art in the field of continuous flow nitration with miniaturized devices. Although nitration has been one of the oldest and most important unit reactions, the advent of miniaturized devices has paved the way for new opportuni- ties to reconsider the conventional approach for exothermic and selectivity sensitive nitration reactions. Four different approaches to flow nitration with microreactors are presented herein and discussed in view of their advantages, limitations and applicability of the information towards scale-up. Selected recent patents that disclose scale-up methodologies for continuous flow nitration are also briefly reviewed. Review 1 Introduction Nitration of aromatics is one of the oldest and industrially most Based on estimations of 2007 and the proposed world produc- important reactions. A reaction between an organic compound tion capacity, the overall world production of nitric acid in 2012 and a nitrating agent leads to the introduction of a nitro group is assumed to be close to 78 Mi TPA, of which 85% is used for onto a carbon, nitrogen or oxygen atom of that organic com- the production of ammonium nitrate as fertilizer and 6% for pound [1]. -

A Fundamental Study of the Alkaline Sulfide Leaching of Gold
The European Journal of Mineral Processing and Environmental Protection Vol.3, No.3, 1303-0868, 2003, pp. 336-343 A fundamental study of the alkaline sulfide leaching of gold M.I. Jeffrey1* and C.G Anderson2 1 Department of Chemical Engineering, Monash University, Vic., 3800, Australia 2 The Center for Advanced Mineral and Metallurgical Processing, Montana Tech of the University of Montana, Butte, Montana, 59701, USA Received 19 September 2002; accepted 23 September 2003 ABSTRACT Due to the increasing concerns over the emission of sulfur dioxide from roasting and smelting, there has been an increased interest in pressure oxidation as a means of treating gold bearing ores and concentrates. One of the problems with the partial oxidation of the sulfide host matrix to form elemental sulfur is that sulfur containing streams are difficult to treat using cyanidation. In the present paper, the alkaline sulfide system was studied as an alternative to cyanide for recovering gold from elemental sulfur. It was found that the dominant lixiviant for gold in this system is sulfide, and polysulfide was ascertained to be the oxidant. The leaching reaction was determined to be chemically controlled, thus highly dependent on temperature. Other variables investigated include the effect of pH, gold purity, and the ratio of sulfide to polysulfides. It was found that each of these variables had a large impact on the system. © 2003 SDU. All rights reserved. Keywords: Hydrometallurgy; Leaching; Gold 1. INTRODUCTION Many gold ores are problematic as they contain metal sulfides, such as pyrite, arsenopyrite and chalcopyrite. These ores are often refractory, as the gold is encapsulated within the sulfide matrix. -

Nibco Chemical Resistance Guide for Valves & Fittings
NIBCO CHEMICAL RESISTANCE GUIDE FOR VALVES & FITTINGS INTRODUCTION liquids into the system. ABS-DWV is very resistant to a wide vari- ety of materials ranging from sewage to commercial household This chemical resistance guide has been compiled to assist the chemical formulations. ABS-DWV is joined by solvent cementing piping system designer in selecting chemical resistant or threading and can easily be connected to steel, copper, or cast materials. The information given is intended as a guide only. Many iron through the use of transition fittings. conditions can affect the material choices. Careful CPVC — Chlorinated Polyvinyl Chloride Class 23447-B, formerly consideration must be given to temperature, pressure and chemi- designated Type IV, Grade 1 conforming to ASTM D-1784, has cal concentrations before a final material can be selected. physical properties at 73°F similar to those of PVC, and its chemi- Thermoplastics’ and elastomers’ physical characteristics are more cal resistance is similar to or generally better than that of PVC. sensitive to temperature than metals. For this reason, a rating chart CPVC, with a design stress of 2000 psi and maximum service has been developed for each. temperature of 210°F, has proven to be an excellent material for MATERIAL RATINGS hot corrosive liquids, hot or cold water distribution, and similar FOR THERMOPLASTICS & ELASTOMERS applications above the temperature range of PVC. CPVC is joined by solvent cementing, threading or flanging. Temp. in °F = “A” rating, maximum temperature which is recommended, resistant under normal PP (Polypropylene) — Type 1 Polypropylene is a polyolefin, which conditions is lightweight and generally high in chemical resistance.