Oxidation of Acetophenone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemistry (55)
156 Chemistry (55) Introduction 3) To expose the students to various emerging According to NCF 2005, the new and new areas of chemistry and apprise them updated curriculum is introduced at +2 stage. with their relevance in their future studies There is a need to provide the sufficient and their applications in various spheres conceptual background of chemistry which will of chemical sciences and technology. help the students to appear for different common 4) To equip students to face various changes entrance test at the state level and the national related to health, nutrition, environment, level. This new syllabus will make them population, weather, industries and competent to meet the challenges of academic agriculture. and professional courses like medicine, 5) To develop problem solving skills in engineering, technology, etc, after the +2 stage. students. The syllabus is comparable to the international 6) To expose the students to different level. processes used in industries and their The syllabus contains areas like physical, technological applications. organic, inorganic, industrial, analytical and 7) To apprise students with interface of polymer chemistry. The upgraded syllabus has chemistry with other disciplines of science taken care of new formulations and such as physics, biology, geology, nomenclature of elements, compounds and engineering, etc. IUPAC units of physical quantities. New nomenclature, symbols and formulations, Std. XI (Theory) fundamental concepts, modern techniques are given importance. Unit 1: Some Basic Concepts of Chemistry Objectives : General Introduction: Importance and The broad objectives of teaching scope of chemistry. Historical approach to Chemistry at Higher Secondary stage are to particulate nature of matter, laws of help the learners : chemical combination, Dalton’s atomic 1) To promote understanding of basic facts theory : concept of elements, atoms and and concepts in chemistry while retaining molecules. -

Download Download
Test for Acetone in Urine 189 AN IMPROVED TEST FOR ACETONE IN URINE. R. E. Lyons and J. T. Brundage, Indiana University. Lieben's test for acetone 1 depends upon the formation of iodoform when potassium iodide, iodine solution, and a few drops of sodium hydroxide solution are added to an acetone containing mixture. The iodoform is recognized by its distinctive odor and, microscopically, by the 1 star, or hexagonal crystals. The test is not specific since both ethyl alcohol and acetic aldehyde also react with these reagents to yield iodo- form. This sometimes leads to erroneous results because of alcohol formed through sugar fermentation in diabetic urine. The difficulty is obviated" by substituting ammonium hydroxide for the caustic alkali as proposed by Gunning'5 in a modification of the Lieben Test. In either test the reaction is much more sensitive if a urine dis- tillate is used. The distillation not only frees the acetone from non- volatile interfering substances, but converts some acetonacetic (di-acetic) 4 acid, if present, into acetone. Protein interferes and, if present, the separation of acetone by distillation or aeration is necessary. M. KohlthofF' states that 100 mg. each of potassium iodide and chlor- amine T, 10-20 drops of 4N ammonium hydroxide and 10 cc. of a solu- tion of one part acetone in 10,000 parts of 2 per cent ethyl alcohol when warmed to 60 °C. gave an iodoform precipitate in two hours. The object of our investigation has been to determine (a) whether this reaction could be applied as a specific test for acetone in urine, (b) what urinary constituents or preservatives interfere, (c) if the necessity of distillation, or aeration, of the urine could be dispensed with, and (d) the conditions for attaining the maximum sensitiveness of the reaction. -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Acetophenone Sigma Aldrich.Pdf
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/27/2010 Print Date 07/08/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Acetophenone Product Number : A10701 Brand : Sigma-Aldrich Company : Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO 63103 USA Telephone : +1 800-325-5832 Fax : +1 800-325-5052 Emergency Phone # : (314) 776-6555 2. HAZARDS IDENTIFICATION Emergency Overview OSHA Hazards Combustible Liquid, Harmful by ingestion., Irritant GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H227 Combustible liquid H302 Harmful if swallowed. H318 Causes serious eye damage. Precautionary statement(s) P210 Keep away from heat/sparks/open flames/hot surfaces. - No smoking. P264 Wash skin thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P280 Wear protective gloves/protective clothing/eye protection/face protection. P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P310 Immediately call a POISON CENTER or doctor/physician. P330 Rinse mouth. P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction. P403 + P235 Store in a well-ventilated place. Keep cool. P501 Dispose of contents/container to an approved waste disposal plant. HMIS Classification Health hazard: 2 Flammability: 2 Physical hazards: 0 NFPA Rating Health hazard: 2 Fire: 2 Reactivity Hazard: 0 Potential Health Effects Sigma-Aldrich - A10701 Page 1 of 6 Inhalation May be harmful if inhaled. -

ACETOPHENONE Method No. PV2003 Target Concentration
ACETOPHENONE Method no. PV2003 Target concentration: 100 ppm (491 mg/m3) Procedure: Samples are collected by drawing a known volume of air through a Tenax GC tube. Samples are desorbed with a solvent mixture of (5:95) Isopropanol:Carbon Disulfide (IPA):(CS2) and analyzed by gas chromatography with a flame ionization detector. Recommended air volume 120 minutes at 0.1 L/min (12 L) and sampling rate: Status of Method: Partially Validated. This method has been only partially evaluated and is presented for information and trial use. July 1982 Wayne Potter Service Branch 1 OSHA Salt Lake Technical Center Salt Lake City UT-84115 1 of 8 1 General Discussion 1.1 Background 1.1.1 History of procedure Recently, the OSHA Analytical Laboratory received a set of field samples that required analysis for acetophenone. The air samples had been collected on charcoal tubes and isopropanol impingers. Desorption studies were done on charcoal tubes using acetone, carbon disulfide, 5:95 isopropanol:carbon disulfide, and methylene chloride as desorbing solvents. The best results were obtained with 5:95 isopropanol:carbon disulfide, but the recovery was only 68%, which indicated that charcoal tubes should not be recommended for the collection of acetophenone air samples. An evaluation of isopropanol impingers was not performed because it was preferable to find an adsorbent procedure for sample collection. NIOSH method 291 for α -chloroacetophenone uses Tenax GC tubes. The Tenax GC tubes were tried for acetophenone, and appeared to be a suitable method of collection. 1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.) Acetophenone is a narcotic in high concentrations (Ref. -

Laboratory 23: Properties of Aldehydes and Ketones
Laboratory 23: Properties of Aldehydes and Ketones Introduction Aldehydes and Ketones represent an important class of organic molecules containing a carbonyl carbon. In this experiment you will study the chemical properties of aldehydes and ketones. Solubility in water, and organic solvents, combustibility, and reactivity with various chemical reagents will be examined. Discussion Structure of Aldehydes and Ketones Aldehydes and Ketones are organic compounds containing a carbonyl carbon (R−C−O−R') (Figure 1 below) functional group. Carboxylic Acids and Esters also contain a carbonyl carbon, and will be explored in a future experiment. The carbonyl carbon is a polar group with the carbon having a slight excess of positive charge and the oxygen atom having a slight excess of negative charge. Chemical Properties Aldehydes and ketones are created by the mild oxidation of primary and secondary alcohols. One such method to oxidize alcohols is with copper (II) oxide. Upon heading, copper wire (Cu0) in an open flame leads to the formation of copper (II) oxide. The copper (II) oxide is then reacted with an alcohol to form an aldehyde or ketone, copper (I) oxide and water. [O] Primary Alcohol −−! Aldehyde [O] Secondary Alcohol −−! Ketone Chemically aldehydes and ketones both contain a carbonyl carbon and thus have similar chemical reactivities. However, aldehydes are more susceptible to oxidation because of the hydrogen atom attached to the carbonyl group. This is the basis for distinguishing between these two classes of compounds. Several tests are useful for differentiating between aldehydes and ketones. The first test is referred to as the Tollens' or Silver Mirror test. -
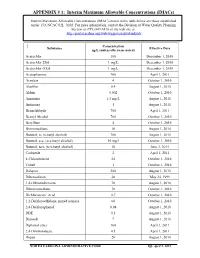
APPENDIX # 1: Interim Maximum Allowable Concentrations (Imacs)
APPENDIX # 1: Interim Maximum Allowable Concentrations (IMACs) Interim Maximum Allowable Concentrations (IMAC) shown in the table below are those established under 15A NCAC 02L .0202. For more information, contact the Division of Water Quality Planning Section at (919) 807-6416 or the web site at: http://portal.ncdenr.org/web/wq/ps/csu/gwstandards Concentration Substance Effective Date ug/L (unless otherwise noted) Acetochlor 100 December 1, 2010 Acetochlor ESA 1 mg/L December 1, 2010 Acetochlor OXA 1 mg/L December 1, 2010 Acetophenone 700 April 1, 2011 Acrolein 4 October 1, 2010 Alachlor 0.4 August 1, 2010 Aldrin 0.002 October 1, 2010 Ammonia 1.5 mg/L August 1, 2010 Antimony 1 August 1, 2010 Benzaldehyde 700 April 1, 2011 Benzyl Alcohol 700 October 1, 2010 Beryllium 4 October 1, 2010 Bromomethane 10 August 1, 2010 Butanol, n- (n-butyl alcohol) 700 August 1, 2010 Butanol, sec- (sec-butyl alcohol) 10 mg/l October 1, 2010 Butanol, tert- (tert-butyl alcohol) 10 June 1, 2011 Carbazole 2 April 1, 2011 4-Chlorotoluene 24 October 1, 2010 Cobalt 1 October 1, 2010 Dalapon 200 August 1, 2010 Dibenzofuran 28 May 24, 1999 1,4-Dibromobenzene 70 August 1, 2010 Dibromomethane 70 October 1, 2010 Dichloroacetic Acid 0.7 October 1, 2010 1,2-Dichloroethylene, mixed isomers 60 October 1, 2010 2,4-Dichlorophenol 0.98 August 1, 2010 DDE 0.1 August 1, 2010 Dinoseb 7 August 1, 2010 Diphenyl ether 100 April 1, 2011 2,4-Dinitrotoluene 0.1 April 1, 2011 Diquat 20 August 1, 2010 NORTH CAROLINA ADMINISTRATIVE CODE Eff. -

Pharmaceutical Services Division and the Clinical Research Centre Ministry of Health Malaysia
A publication of the PHARMACEUTICAL SERVICES DIVISION AND THE CLINICAL RESEARCH CENTRE MINISTRY OF HEALTH MALAYSIA MALAYSIAN STATISTICS ON MEDICINES 2008 Edited by: Lian L.M., Kamarudin A., Siti Fauziah A., Nik Nor Aklima N.O., Norazida A.R. With contributions from: Hafizh A.A., Lim J.Y., Hoo L.P., Faridah Aryani M.Y., Sheamini S., Rosliza L., Fatimah A.R., Nour Hanah O., Rosaida M.S., Muhammad Radzi A.H., Raman M., Tee H.P., Ooi B.P., Shamsiah S., Tan H.P.M., Jayaram M., Masni M., Sri Wahyu T., Muhammad Yazid J., Norafidah I., Nurkhodrulnada M.L., Letchumanan G.R.R., Mastura I., Yong S.L., Mohamed Noor R., Daphne G., Kamarudin A., Chang K.M., Goh A.S., Sinari S., Bee P.C., Lim Y.S., Wong S.P., Chang K.M., Goh A.S., Sinari S., Bee P.C., Lim Y.S., Wong S.P., Omar I., Zoriah A., Fong Y.Y.A., Nusaibah A.R., Feisul Idzwan M., Ghazali A.K., Hooi L.S., Khoo E.M., Sunita B., Nurul Suhaida B.,Wan Azman W.A., Liew H.B., Kong S.H., Haarathi C., Nirmala J., Sim K.H., Azura M.A., Asmah J., Chan L.C., Choon S.E., Chang S.Y., Roshidah B., Ravindran J., Nik Mohd Nasri N.I., Ghazali I., Wan Abu Bakar Y., Wan Hamilton W.H., Ravichandran J., Zaridah S., Wan Zahanim W.Y., Kannappan P., Intan Shafina S., Tan A.L., Rohan Malek J., Selvalingam S., Lei C.M.C., Ching S.L., Zanariah H., Lim P.C., Hong Y.H.J., Tan T.B.A., Sim L.H.B, Long K.N., Sameerah S.A.R., Lai M.L.J., Rahela A.K., Azura D., Ibtisam M.N., Voon F.K., Nor Saleha I.T., Tajunisah M.E., Wan Nazuha W.R., Wong H.S., Rosnawati Y., Ong S.G., Syazzana D., Puteri Juanita Z., Mohd. -

DESIGN, SYNTHESIS, CHARACTERIZATION of SOME NEW SUBSTITUTED CHALCONES and STUDIES THEIR ANTIMICROBIAL ACTIVITIES Zakaria H
[Aiube* et al., 5(6): June, 2016] ISSN: 2277-9655 IC™ Value: 3.00 Impact Factor: 4.116 IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY DESIGN, SYNTHESIS, CHARACTERIZATION OF SOME NEW SUBSTITUTED CHALCONES AND STUDIES THEIR ANTIMICROBIAL ACTIVITIES Zakaria H. Aiube*, Ali H. Samir, Israa SH. A- R. Al- Kadi * Department of Chemistry, college of Education for pure Science-Ibn, Al- Haitham, University, Baghdad, Iraq DOI: 10.5281/zenodo.55982 ABSTRACT Eight designed chalcones, named [1(p-benzenesulphonamidophenyl)-3-p-chloro-2-propene-1-one][2], [1(p- benzenesulphonamidophenyl)-3-p-nitro-2-propene-1-one][3], [1(4-Ureido)phenyl-3-p-chlorophenyl-2-propene- 1-one][5], [1(4-Ureido)phenyl-3-p-nitroophenyl-2-propene-1-one][6], [1(4(p-N-methylaminophenyl)azophenyl- 3-p-chlorophenyl-2-propene-1-one][8], [1(4(p-N-methylaminophenyl)azophenyl-3-p-nitroophenyl-2-propene-1- one][9], [1(p-aminophenyl)-3-p-chlorophenyl-2-propene-1-one][10] and [1(p-aminophenyl)-3-p-nitrophenyl-2- propene-1-one][11], were synthesised by condensation of synthesised p-acetylphenylbenzene- sulphonamide, p- acetylphenylurea and p-acetyl-p'-(N-methylamino)azobenzene, with p-chlorobenzaldehyde and p nitrobenzaldehyde in basic media respectively. All synthesised compounds are characterized by its melting points, FTIR, 1H NMR, 13C NMR and Mass spectral analysis. All synthesised compounds are examined their antimicrobial activities against Gram-Ve bactria (Serratia marcescens, Pseudmonas aeroginosa) and Gram+Ve bacterial (Stphylococcus aureus, Streptococcus pyogenes), and Candida albicans fungi. Result showed good to moderate inhibition effect against some bacteria and fungi, in comparison with some pharmaceutical antibiotic and antifungal treatments like Cephalexin, Amoxicillin, Tetracycline, Lincomycine, Nystatine and Fluconazole respectively. -

Safety Data Sheet Iodoform Compound Paint
SAFETY DATA SHEET IODOFORM COMPOUND PAINT 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COM PANY: PRODUCT NAME: IODOFORM COMPOUND PAINT PART No.: M028 SUPPLIER: J M Loveridge plc Southbrook Road, Southampton Hampshire SO15 1BH Tel: 023 8022 2008 Fax: 023 8022 2117 2. COMPOSITION/INFORMATION ON INGREDIENTS: NAME CONTENT CAS No.: EINECS Nr.: CLASSIFICATION DIETHYL ETHER 60-100 % 60-29-7 200-467-2 Xn ,Fx R-12, 19, 22, 66, 67 IODOFORM 10-30 % 75-47-8 200-874-5 Xn R-20/21/22, 36 The Full Text for all R-Phrases are Displayed in Section 16 3. HAZARDS IDENTIFICATION: Extremely flammable. Harmful if swallowed. Repeated exposure may cause skin dryness or cracking. Vapours may cause drowsiness and dizziness. 4. FIRST AID MEASURES: GENERAL: IN ALL CASES OF DOUBT OR WHEN SYMPTOMS PERSIST, ALWAYS SEEK MEDICAL ATTENTION IN H A LA T IO N : Move affected person from exposure. If recovery not rapid or complete seek medical attention. If breathing stops, provide artificial respiration. Keep affected person warm and at rest. IN G E ST IO N: DO NOT INDUCE VOMITING. In case of spontaneous vomiting, be sure that vomit can freely drain because of danger of suffocation. Only when conscious, rinse mouth with plenty of water and give plenty of water to drink - (approx 500ml). Keep patient at rest and obtain medical attention. SKIN: Remove contaminated clothing. Wash affected area with plenty of soap and water. If 1/5 10178 - IODOFORM COMPOUND PAINT symptoms occur or persist, obtain medical attention. Launder clothing before re-use. EYES: Rinse immediately with plenty of water for at least 5 minutes while lifting the eye lids. -

Haloform Reaction - Wikipedia
6/13/2020 Haloform reaction - Wikipedia Haloform reaction The haloform reaction is a chemical reaction where a haloform Haloform reaction (CHX , where X is a halogen) is produced by the exhaustive 3 Named after Adolf Lieben halogenation of a methyl ketone (RCOCH3, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a Reaction type Substitution base.[1][2][3] The reaction can be used to transform acetyl groups into reaction carboxyl groups or to produce chloroform (CHCl3), bromoform Identifiers (CHBr3), or iodoform (CHI3) and also cyanide. Organic haloform-reaction Chemistry Portal Contents Mechanism Scope Applications Laboratory scale Industrially As a by-product of water chlorination History References Mechanism In the first step, the halogen disproportionates in the presence of hydroxide to give the halide and hypohalite (example with bromine, but reaction is the same in case of chlorine and iodine; one should only substitute Br for Cl or I): If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite: If a methyl ketone is present, it reacts with the hypohalite in a three-step process: 1. Under basic conditions, the ketone undergoes keto-enol tautomerization. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge). https://en.wikipedia.org/wiki/Haloform_reaction#Iodoform_reaction 1/5 6/13/2020 Haloform reaction - Wikipedia 2. When the α(alpha) position has been exhaustively halogenated, the molecule undergoes a nucleophilic − acyl substitution by hydroxide, with CX3 being the leaving group stabilized by three electron- − withdrawing groups. -

Iodoform Packing
Created by the British Columbia Provincial Nursing Skin and Wound Committee in collaboration with the Wound Clinicians from / Skin and Wound Product Information Sheet Iodoform Packing Classification Antimicrobial/Antiseptic: Iodine Key Points A ravel-resistant strip gauze packing impregnant with Iodoform, an antiseptic effective against pseudomonas. In the presence of exudate, iodoform breaks down to release Iodine (96%) and therefore should be used with caution. The packing’s colour may be opaque to yellowish and may have an oily appearance, as a small amount of oil may have been use to aid in easier removal of product from the container. Should be packed dry. Indications Use under the direction of a Physician, NP, NSWOC/Wound Clinician: o For wounds with or without undermining, sinus tract/tunnels which have signs and symptoms (S&S) of, or are at risk for, local wound infection. Precautions May cause some discomfort/pain, especially when first applied. Avoid using before and after radio-iodine diagnostic tests Make Physician/NP aware of Iodine usage for clients: o Taking lithium, as Iodine may increase the possibility of hypothyroidism when used in combination with lithium. Blood work should be monitored on a regular basis. o With renal impairment, as poor renal function is thought to be a factor in increased iodine levels in serum and urine with prolonged use and use in large wounds. o With thyroid disorders, as they are more susceptible to thyroid metabolism changes in long-term therapy. Thyroid function should be monitored if large areas are being treated for a prolonged period of time.