Tracing Fluid Transfers in Subduction Zones: an Integrated 18 2 Thermodynamic and Δ O Fractionation Modelling Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

Fire Retardancy of Polypropylene/Kaolinite Composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta
Fire retardancy of polypropylene/kaolinite composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta To cite this version: Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta. Fire retardancy of polypropylene/kaolinite composites. Polymer Degradation and Stability, Elsevier, 2016, 129, pp.260-267. 10.1016/j.polymdegradstab.2016.05.003. hal-02906432 HAL Id: hal-02906432 https://hal.archives-ouvertes.fr/hal-02906432 Submitted on 26 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Fire retardancy of polypropylene/kaolinite composites * Marcos Batistella a, c, , Belkacem Otazaghine b, Rodolphe Sonnier b, Carlos Petter c, Jose-Marie Lopez-Cuesta b a Federal University of Santa Catarina, R. Eng. Agronomico^ Andrei Cristian Ferreira, s/n e Trindade, Florianopolis, SC, CEP 88040-900, Brazil b Ecole des Mines d’Ales, Centre des Materiaux (C2MA) e Pole^ Materiaux Polymeres Avances, 6 Avenue de Clavieres, 30319, Ales Cedex, France c Federal University of Rio Grande do Sul, Av. Bento Gonçalves, 9500, Porto Alegre, CEP 91501-970, Brazil abstract In this study the influence of surface modification of kaolinite with trisilanolisooctyl Polyhedral Oligo- SilSesquioxane (POSS) in polypropylene composites was evaluated in terms of thermal stability and fire retardancy and compared with talc. -

NMAM 9000: Asbestos, Chrysotile By
ASBESTOS, CHRYSOTILE by XRD 9000 MW: ~283 CAS: 12001-29-5 RTECS: CI6478500 METHOD: 9000, Issue 3 EVALUATION: FULL Issue 1: 15 May 1989 Issue 3: 20 October 2015 EPA Standard (Bulk): 1% by weight PROPERTIES: Solid, fibrous mineral; conversion to forsterite at 580 °C; attacked by acids; loses water above 300 °C SYNONYMS: Chrysotile SAMPLING MEASUREMENT BULK TECHNIQUE: X-RAY POWDER DIFFRACTION SAMPLE: 1 g to 10 g ANALYTE: Chrysotile SHIPMENT: Seal securely to prevent escape of asbestos PREPARATION: Grind under liquid nitrogen; wet-sieve SAMPLE through 10 µm sieve STABILITY: Indefinitely DEPOSIT: 5 mg dust on 0.45 µm silver membrane BLANKS: None required filter ACCURACY XRD: Copper target X-ray tube; optimize for intensity; 1° slit; integrated intensity with RANGE STUDIED: 1% to 100% in talc [1] background subtraction BIAS: Negligible if standards and samples are CALIBRATION: Suspensions of asbestos in 2-propanol matched in particle size [1] RANGE: 1% to 100% asbestos OVERALL PRECISION ( ): Unknown; depends on matrix and ESTIMATED LOD: 0.2% asbestos in talc and calcite; 0.4% concentration asbestos in heavy X-ray absorbers such as ferric oxide ACCURACY: ±14% to ±25% PRECISION ( ): 0.07 (5% to 100% asbestos); 0.10 (@ 3% asbestos); 0.125 (@ 1% asbestos) APPLICABILITY: Analysis of percent chrysotile asbestos in bulk samples. INTERFERENCES: Antigorite (massive serpentine), chlorite, kaolinite, bementite, and brushite interfere. X-ray fluorescence and absorption is a problem with some elements; fluorescence can be circumvented with a diffracted beam monochromator, and absorption is corrected for in this method. OTHER METHODS: This is NIOSH method P&CAM 309 [2] applied to bulk samples only, since the sensitivity is not adequate for personal air samples. -

A108-316 (10/10/16)
American Industrial Hygiene Association Bulk Asbestos Proficiency Analytical Testing Program Results of Round A108-316 10/10/2016 John Herrock Laboratory ID Number Total Penalty Points 0 University of Louisiana, Monroe - Dept of 213022 Round Status P Toxicology Program Status P 700 University Ave. Monroe, LA 71209 UNITED STATES Lot Designation\Sample ID Numbers A) 1761 B) 2702 C) 1897 D) 4134 Analysis Results from Laboratory Number 213022 Asbestos (%) CHRY (3) ANTH(22) NONE CHRY (1) Other Fibrous Materials (%) FBGL (1) Nonfibrous Material (%) ACID (52) OTHR (55) ACID (60) OTHR (60) MICA (33) MICA (11) OTHR (38) ACID (29) Penalty Points Assessed 0 0 0 0 Analysis Results from Reference Laboratory One Asbestos (%) CHRY(5.8) ANTH (12) NONE CHRY (3.8) ACTN (0.1) Other Fibrous Materials (%) CELL (0.1) OTHR *1 (0.1) CELL (1) Nonfibrous Material (%) MICA (45) OTHR *2(87.9) OTHR *3 (35) PERL (20) CASO (49) OTHR *4 (65) OTHR *5 (20) OTHR *6 (55.2) Analysis Results from Reference Laboratory Two Asbestos (%) CHRY (2.5) ANTH (28) (0) CHRY(3.5%) TREM(trace) Other Fibrous Materials (%) FBGL (trace) Nonfibrous Material (%) OTHR *7 (60) OTHR *9 (24) OTHR *11 (80) OTHR *14 (20) OTHR *8(37.5) OTHR *10 (48) OTHR *12 (18) OTHR *15(76.5) OTHR *13 (2) Analysis Results from RTI International Asbestos (%) CHRY (4) ANTH (28) NONE CHRY (3) ACTN (Tra) Other Fibrous Materials (%) OTHR *16(Tra) POLY (Tra) CELL (1) OTHR *17(Tra) Nonfibrous Material (%) MICA (29) OTHR *18 (53) CACO (89) OTHR *22 (28) CASO (67) OTHR *19 (19) OTHR *20 (9) PERL (45) OTHR *21 (2) OTHR *23 -

40 Common Minerals and Their Uses
40 Common Minerals and Their Uses Aluminum Beryllium The most abundant metal element in Earth’s Used in the nuclear industry and to crust. Aluminum originates as an oxide called make light, very strong alloys used in the alumina. Bauxite ore is the main source aircraft industry. Beryllium salts are used of aluminum and must be imported from in fluorescent lamps, in X-ray tubes and as Jamaica, Guinea, Brazil, Guyana, etc. Used a deoxidizer in bronze metallurgy. Beryl is in transportation (automobiles), packaging, the gem stones emerald and aquamarine. It building/construction, electrical, machinery is used in computers, telecommunication and other uses. The U.S. was 100 percent products, aerospace and defense import reliant for its aluminum in 2012. applications, appliances and automotive and consumer electronics. Also used in medical Antimony equipment. The U.S. was 10 percent import A native element; antimony metal is reliant in 2012. extracted from stibnite ore and other minerals. Used as a hardening alloy for Chromite lead, especially storage batteries and cable The U.S. consumes about 6 percent of world sheaths; also used in bearing metal, type chromite ore production in various forms metal, solder, collapsible tubes and foil, sheet of imported materials, such as chromite ore, and pipes and semiconductor technology. chromite chemicals, chromium ferroalloys, Antimony is used as a flame retardant, in chromium metal and stainless steel. Used fireworks, and in antimony salts are used in as an alloy and in stainless and heat resisting the rubber, chemical and textile industries, steel products. Used in chemical and as well as medicine and glassmaking. -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Talc and Pyrophyllite
TALC AND PYROPHYLLITE By Robert L. Virta Domestic survey data and tables were prepared by Raymond I. Eldridge III, statistical assistant, and the world production table was prepared by Glenn J. Wallace, international data coordinator. The mineral talc is a hydrous magnesium silicate. A massive recommended against listing asbestiform talc and talcose rock is called steatite, and an impure massive variety is nonasbestiform talc in its 10th report on carcinogens (U.S. known as soapstone. Talc is used commercially because of its Department of Health and Human Services, Public Health fragrance retention, luster, purity, softness, and whiteness. Service, 2001; National Paint and Coatings Association, Other commercially important properties of talc are its chemical January 2001, NTP skips over talc, accessed January 8, 2001, at inertness, high dielectric strength, high thermal conductivity, URL http://www.paint.org/ind_issue/current/jan/issue05.htm). low electrical conductivity, and oil and grease adsorption. In 2000, the U.S. Department of Defense authorized the Major markets for talc are ceramics, paint, paper, and plastics. disposal of 907 metric tons (t) of block and lump talc, which is Pyrophyllite is a hydrous aluminum silicate with a structure the entire uncommitted inventory in that category, from the similar to talc. Such properties as chemical inertness, high National Defense Stockpile. dielectric strength, high melting point, and low electrical conductivity make it useful for ceramic and refractory Production applications. Talc.—In 2000, seven companies operating nine mines in five Legislation and Government Programs States produced soapstone, steatite, and talc. All were open pit mines. The producers were, in decreasing order of production, The National Toxicology Program (NTP) of the U.S. -

Swelling Capacity of Mixed Talc-Like/Stevensite Layers in White/Green Clay
This is a preprint, the final version is subject to change, of the American Mineralogist (MSA) Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2020-6984 1 1 Plagcheck: no concerns 2 Tables?: 3 small 3 Word Count: ~9,100 4 Prod notes: make sure tables in file before RE 5 6 7 8 Swelling capacity of mixed talc-like/stevensite layers in white/green clay 9 infillings (‘deweylite’/‘garnierite’) from serpentine veins of faulted 10 peridotites, New Caledonia 11 REVISION 2 12 Lionel FONTENEAU 1, Laurent CANER 2*, Sabine PETIT 2, Farid JUILLOT 3, Florian 13 PLOQUIN 3, Emmanuel FRITSCH 3 14 15 1Corescan Pty Ltd, 1/127 Grandstand Road, 6104 Ascot, WA, Australia 16 2 Université de Poitiers, Institut de Chimie des Milieux et Matériaux de Poitiers, IC2MP UMR 17 7285 CNRS, 5 rue Albert Turpain, TSA51106, 86073 Poitiers cedex 9, France 18 * Corresponding author, e-mail: [email protected] 19 3 Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie (IMPMC), Sorbonne 20 Universités – Université Pierre et Marie Curie UPMC, UMR CNRS 7590, Museum National 21 d’Histoire Naturelle, UMR IRD 206, 101 Promenade Roger Laroque, Anse Vata, 98848, 22 Nouméa, New Caledonia 23 24 25 Abstract: White (Mg-rich) and green (Ni-rich) clay infillings (‘deweylite’/‘garnierite’) found 26 in serpentine veins of faulted peridotite formations from New Caledonia consist of an intimate 27 mixture of fine-grained and poorly ordered 1:1 and 2:1 layer silicates, commonly referred to 28 as non-expandable serpentine-like (SL) and talc-like (TL) minerals. -

Commodities, Part 5 Strontium, Sodium Sulfate, Trona (Soda Ash), Talc, Lithium, Summary Comments Safety Reminders
ME571/GEO571 Geology of Industrial Minerals Spring 2018 Commodities, Part 5 strontium, sodium sulfate, trona (soda ash), talc, lithium, summary comments Safety Reminders Commodity presentations—send me your powerpoints April 28 AIPG meeting and Field trip in afternoon (perlite mine or carbonatites) Research Projects presentation April 30 Finals, written Project due May 4 No class May 7 Strontium Strontium—introduction • Sr • 15th abundant element • does not occur naturally as an element, in compounds • No production in the United States since 1959 • celestite or celestine SrSO4 (same structure as barite) 56.4% Sr • strontianite SrCO3, 70.1% Sr Celesitite http://www.zeuter.com/~tburden Strontianite http://www.zeuter.com/~tburden Strontium and strontianite are named after Stronian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank A critical mineral Strontium—uses • faceplate glass of color television picture tubes, 77% • ferrite ceramic magnets, 8% • pyrotechnics and signals, 9% – fireworks (red flame) – flares • other applications, 6% – refining zinc – optical materials Strontium—production USGS Mineral Yearbooks metric tons Strontium—geology • association with rocks deposited by the evaporation of sea water (evaporites) • igneous rocks • Brines • Barite and calcite must be removed— costly Sodium sulfate Sodium sulfate—introduction • disodium sulfate (Na2SO4), • inorganic chemical • Thenardite Na2SO4 • Hanksite Na22K(SO4)9(CO3)2Cl • Glauberite Na2Ca(SO4)2 Sodium sulfate—uses -

The Cordierite Formation in Mechanically Activated Talc-Kaoline-Alumina-Basalt-Quartz Ceramic System D
Vol. 127 (2015) ACTA PHYSICA POLONICA A No. 4 Proceedings of the 4th International Congress APMAS2014, April 24-27, 2014, Fethiye, Turkey The Cordierite Formation in Mechanically Activated Talc-Kaoline-Alumina-Basalt-Quartz Ceramic System D. Kirsever*, N. Karakus, N. Toplan, H.O. Toplan Sakarya University, Metalurgy and Materials Engineering, 54187 Sakarya, Turkey The powder mixtures of Talc-Kaolinite-Alumina-Basalt-Quartz were mechanically activated in a planetary ball mill for 1 hour. The structural alterations and thermal behaviour of the powder mixture were examined by X-ray diraction (XRD), scanning electron microscopy (SEM) and thermal analysis (TG-DTA). The results showed that the mechanical activation led to amorphisation and decreased the temperature of cordierite formation. DOI: 10.12693/APhysPolA.127.1042 PACS: 81.05.Je 1. Introduction a high-energy ball mill. In this process, a small quantity of the blended elemental powder mixture is loaded into a Cordierite, which chemical composition is container, along with the grinding media, and the whole 2MgO 2Al O 5SiO , is one of the phases of the · 2 3· 2 mass is agitated at a high speed for a predetermined ternary MgOSiO Al O system, along with mullite, 2 2 3 length of time [5]. The mechano-activation treatment cristobalite, tridymite, enstatite, forsterite, sapphirine, might promote: the amorphization of treated material, etc. Cordierite-based materials have a great importance noticeable change of the microstructure, size and shape in modern technology due to their excellent properties, of particles, etc. Furthermore, ultra-ne grinding kinetic such as low thermal expansion coecient, high refrac- investigation indicates the mechano-chemical reduction toriness, low dielectric constant, high thermal shock of the original particles of talc/mica, which appears to resistance and good mechanical properties [1]. -

A RARE-ALKALI BIOTITE from KINGS MOUNTAIN, NORTH CAROLINA1 Fnanr L
A RARE-ALKALI BIOTITE FROM KINGS MOUNTAIN, NORTH CAROLINA1 FnaNr L. Hnss2 arqn Ror-r.rx E. SrrvrNs3 Severalyears ago, after Judge Harry E. Way of Custer, South Dakota, had spectroscopically detected the rare-alkali metals in a deep-brown mica from a pegmatite containing pollucite and lithium minerals, in Tin Mountain, 7 miles west of Custer, another brown mica was collected, which had developed notably in mica schist at its contact with a similar mass of pegmatite about one half mile east of Tin Mountai". J. J. Fahey of the United States GeolgoicalSurvey analyzed the mica, and it proved to contain the rare-alkali metalsaand to be considerably difierent from any mica theretofore described. Although the cesium-bearing minerals before known (pollucite, lepidolite, and beryl) had come from the zone of highest temperature in the pegmatite, the brown mica was from the zone of lowest temperature. The occurrence naturally suggestedthat where dark mica was found developed at the border of a pegmatite, especially one carrying lithium minerals, it should be examined for the rare-alkali metals. As had been found by Judge Way, spectroscopictests on the biotite from Tin Moun- tain gave strong lithium and rubidium lines, and faint cesium lines. Lithium lines were shown in a biotite from the border of the Morefield pegmatite, a mile south of Winterham, Virginia, but rubidium and cesium w'erenot detected. $imilarly placed dark micas from Newry and Hodgeon HiII, near Buckfield, Maine, gave negative results. They should be retested. Tests by Dr. Charles E. White on a shiny dark mica from the Chestnut FIat pegmatite near Spruce Pine, North Carolina, gave strong lithium and weaker cesium lines. -
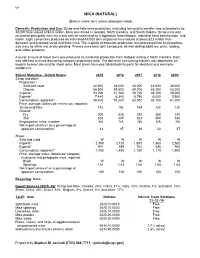
Mica Data Sheet
108 MICA (NATURAL) (Data in metric tons unless otherwise noted) Domestic Production and Use: Scrap and flake mica production, excluding low-quality sericite, was estimated to be 38,000 tons valued at $4.6 million. Mica was mined in Georgia, North Carolina, and South Dakota. Scrap mica was recovered principally from mica and sericite schist and as a byproduct from feldspar, industrial sand beneficiation, and kaolin. Eight companies produced an estimated 63,000 tons of ground mica valued at about $22 million from domestic and imported scrap and flake mica. The majority of domestic production was processed into small-particle- size mica by either wet or dry grinding. Primary uses were joint compound, oil-well-drilling additives, paint, roofing, and rubber products. A minor amount of sheet mica was produced as incidental production from feldspar mining in North Carolina. Data was withheld to avoid disclosing company proprietary data. The domestic consuming industry was dependent on imports to meet demand for sheet mica. Most sheet mica was fabricated into parts for electrical and electronic equipment. Salient Statistics—United States: 2015 2016 2017 2018 2019e Scrap and flake: Production:1 Sold and used 32,600 28,000 40,000 44,000 38,000 Ground 65,800 59,500 69,700 65,300 63,000 Imports2 33,200 31,500 29,700 28,100 29,000 Exports3 7,440 6,340 6,790 6,000 5,900 Consumption, apparent4 58,400 53,200 62,900 66,100 61,000 Price, average, dollars per metric ton, reported: Scrap and flake 142 152 165 122 120 Ground: Dry 305 320 292 308 310 Wet 423 435 424 454 480 Employment, mine, number NA NA NA NA NA Net import reliance5 as a percentage of apparent consumption 44 47 36 33 37 Sheet: Sold and used W W W W W Imports6 2,390 2,120 1,850 1,860 2,500 Exports7 911 689 704 686 950 Consumption, apparent5 1,480 1,430 1,150 1,170 1,600 Price, average value, dollars per kilogram, muscovite and phlogopite mica, reported: Block W W W W W Splittings 1.61 1.61 1.66 1.65 1.65 Net import reliance5 as a percentage of apparent consumption 100 100 100 100 100 Recycling: None.