The Cordierite Formation in Mechanically Activated Talc-Kaoline-Alumina-Basalt-Quartz Ceramic System D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fire Retardancy of Polypropylene/Kaolinite Composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta
Fire retardancy of polypropylene/kaolinite composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta To cite this version: Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta. Fire retardancy of polypropylene/kaolinite composites. Polymer Degradation and Stability, Elsevier, 2016, 129, pp.260-267. 10.1016/j.polymdegradstab.2016.05.003. hal-02906432 HAL Id: hal-02906432 https://hal.archives-ouvertes.fr/hal-02906432 Submitted on 26 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Fire retardancy of polypropylene/kaolinite composites * Marcos Batistella a, c, , Belkacem Otazaghine b, Rodolphe Sonnier b, Carlos Petter c, Jose-Marie Lopez-Cuesta b a Federal University of Santa Catarina, R. Eng. Agronomico^ Andrei Cristian Ferreira, s/n e Trindade, Florianopolis, SC, CEP 88040-900, Brazil b Ecole des Mines d’Ales, Centre des Materiaux (C2MA) e Pole^ Materiaux Polymeres Avances, 6 Avenue de Clavieres, 30319, Ales Cedex, France c Federal University of Rio Grande do Sul, Av. Bento Gonçalves, 9500, Porto Alegre, CEP 91501-970, Brazil abstract In this study the influence of surface modification of kaolinite with trisilanolisooctyl Polyhedral Oligo- SilSesquioxane (POSS) in polypropylene composites was evaluated in terms of thermal stability and fire retardancy and compared with talc. -

Talc and Pyrophyllite
TALC AND PYROPHYLLITE By Robert L. Virta Domestic survey data and tables were prepared by Raymond I. Eldridge III, statistical assistant, and the world production table was prepared by Glenn J. Wallace, international data coordinator. The mineral talc is a hydrous magnesium silicate. A massive recommended against listing asbestiform talc and talcose rock is called steatite, and an impure massive variety is nonasbestiform talc in its 10th report on carcinogens (U.S. known as soapstone. Talc is used commercially because of its Department of Health and Human Services, Public Health fragrance retention, luster, purity, softness, and whiteness. Service, 2001; National Paint and Coatings Association, Other commercially important properties of talc are its chemical January 2001, NTP skips over talc, accessed January 8, 2001, at inertness, high dielectric strength, high thermal conductivity, URL http://www.paint.org/ind_issue/current/jan/issue05.htm). low electrical conductivity, and oil and grease adsorption. In 2000, the U.S. Department of Defense authorized the Major markets for talc are ceramics, paint, paper, and plastics. disposal of 907 metric tons (t) of block and lump talc, which is Pyrophyllite is a hydrous aluminum silicate with a structure the entire uncommitted inventory in that category, from the similar to talc. Such properties as chemical inertness, high National Defense Stockpile. dielectric strength, high melting point, and low electrical conductivity make it useful for ceramic and refractory Production applications. Talc.—In 2000, seven companies operating nine mines in five Legislation and Government Programs States produced soapstone, steatite, and talc. All were open pit mines. The producers were, in decreasing order of production, The National Toxicology Program (NTP) of the U.S. -

Swelling Capacity of Mixed Talc-Like/Stevensite Layers in White/Green Clay
This is a preprint, the final version is subject to change, of the American Mineralogist (MSA) Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2020-6984 1 1 Plagcheck: no concerns 2 Tables?: 3 small 3 Word Count: ~9,100 4 Prod notes: make sure tables in file before RE 5 6 7 8 Swelling capacity of mixed talc-like/stevensite layers in white/green clay 9 infillings (‘deweylite’/‘garnierite’) from serpentine veins of faulted 10 peridotites, New Caledonia 11 REVISION 2 12 Lionel FONTENEAU 1, Laurent CANER 2*, Sabine PETIT 2, Farid JUILLOT 3, Florian 13 PLOQUIN 3, Emmanuel FRITSCH 3 14 15 1Corescan Pty Ltd, 1/127 Grandstand Road, 6104 Ascot, WA, Australia 16 2 Université de Poitiers, Institut de Chimie des Milieux et Matériaux de Poitiers, IC2MP UMR 17 7285 CNRS, 5 rue Albert Turpain, TSA51106, 86073 Poitiers cedex 9, France 18 * Corresponding author, e-mail: [email protected] 19 3 Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie (IMPMC), Sorbonne 20 Universités – Université Pierre et Marie Curie UPMC, UMR CNRS 7590, Museum National 21 d’Histoire Naturelle, UMR IRD 206, 101 Promenade Roger Laroque, Anse Vata, 98848, 22 Nouméa, New Caledonia 23 24 25 Abstract: White (Mg-rich) and green (Ni-rich) clay infillings (‘deweylite’/‘garnierite’) found 26 in serpentine veins of faulted peridotite formations from New Caledonia consist of an intimate 27 mixture of fine-grained and poorly ordered 1:1 and 2:1 layer silicates, commonly referred to 28 as non-expandable serpentine-like (SL) and talc-like (TL) minerals. -

Commodities, Part 5 Strontium, Sodium Sulfate, Trona (Soda Ash), Talc, Lithium, Summary Comments Safety Reminders
ME571/GEO571 Geology of Industrial Minerals Spring 2018 Commodities, Part 5 strontium, sodium sulfate, trona (soda ash), talc, lithium, summary comments Safety Reminders Commodity presentations—send me your powerpoints April 28 AIPG meeting and Field trip in afternoon (perlite mine or carbonatites) Research Projects presentation April 30 Finals, written Project due May 4 No class May 7 Strontium Strontium—introduction • Sr • 15th abundant element • does not occur naturally as an element, in compounds • No production in the United States since 1959 • celestite or celestine SrSO4 (same structure as barite) 56.4% Sr • strontianite SrCO3, 70.1% Sr Celesitite http://www.zeuter.com/~tburden Strontianite http://www.zeuter.com/~tburden Strontium and strontianite are named after Stronian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank A critical mineral Strontium—uses • faceplate glass of color television picture tubes, 77% • ferrite ceramic magnets, 8% • pyrotechnics and signals, 9% – fireworks (red flame) – flares • other applications, 6% – refining zinc – optical materials Strontium—production USGS Mineral Yearbooks metric tons Strontium—geology • association with rocks deposited by the evaporation of sea water (evaporites) • igneous rocks • Brines • Barite and calcite must be removed— costly Sodium sulfate Sodium sulfate—introduction • disodium sulfate (Na2SO4), • inorganic chemical • Thenardite Na2SO4 • Hanksite Na22K(SO4)9(CO3)2Cl • Glauberite Na2Ca(SO4)2 Sodium sulfate—uses -

Talc Processing
11.26 Talc Processing 11.26.1 Process Description1-9 . Talc, which is a soft, hydrous magnesium silicate (3Mg0 4Si02 H20), is used in a wide range of industries including the manufacture of ceramics, paints, paper, and asphalt roofing. The end-uses for talc are determined by variables such as chemical and mineralogical composition, particle size and shape, specific gravity, hardness, and color. There is no Source Classification Code (SCC) for the source category. Over 95 percent of the talc ore produced in the United States comes from open-pit mines. Mining operations usually consist of conventional drilling and blasting methods. Figure 11.26-1 is a process flow diagram for a typical domestic talc plant. Talc ore generally is hauled to the plant by truck from a nearby mine. The ore is crushed, typically in a jaw crusher, and screened. The coarse (oversize) material then is returned to the crusher. Rotary dryers may be used to dry the material. Secondary grinding is achieved with pebble mills or roller mills, producing a product that is 44 to 149 micrometers ( m) (325 to 100 mesh) in size. Some roller mills are designed to use heated air to dry the material as it is being ground. Hammer mills or steam- or compressed air- powered jet mills may be used to produce additional final products. Air classifiers (separators), generally in closed circuit with the mills, separate the material into coarse, coarse-plus-fine, and fine fractions. The coarse and coarse-plus-fine fractions then are stored as products. The fines may be concentrated using a shaking table (tabling process) to separate product containing small quantities of nickel, iron, cobalt, or other minerals and then may undergo a one-step flotation process. -

What We Know About Subduction Zones from the Metamorphic Rock Record
What we know about subduction zones from the metamorphic rock record Sarah Penniston-Dorland University of Maryland Subduction zones are complex We can learn a lot about processes occurring within active subduction zones by analysis of metamorphic rocks exhumed from ancient subduction zones Accreonary prism • Rocks are exhumed from a wide range of different parts of subduction zones. • Exhumed rocks from fossil subduction zones tell us about materials, conditions and processes within subduction zones • They provide complementary information to observations from active subduction systems Tatsumi, 2005 The subduction interface is more complex than we usually draw Mélange (Bebout, and Penniston-Dorland, 2015) Information from exhumed metamorphic rocks 1. Thermal structure The minerals in exhumed rocks of the subducted slab provide information about the thermal structure of subduction zones. 2. Fluids Metamorphism generates fluids. Fossil subduction zones preserve records of fluid-related processes. 3. Rheology and deformation Rocks from fossil subduction zones record deformation histories and provide information about the nature of the interface and the physical properties of rocks at the interface. 4. Geochemical cycling Metamorphism of the subducting slab plays a key role in the cycling of various elements through subduction zones. Thermal structure Equilibrium Thermodynamics provides the basis for estimating P-T conditions using mineral assemblages and compositions Systems act to minimize Gibbs Free Energy (chemical potential energy) Metamorphic facies and tectonic environment SubduconSubducon zone metamorphism zone metamorphism Regional metamorphism during collision Mid-ocean ridge metamorphism Contact metamorphism around plutons Determining P-T conditions from metamorphic rocks Assumption of chemical equilibrium Classic thermobarometry Based on equilibrium reactions for minerals in rocks, uses the compositions of those minerals and their thermodynamic properties e.g. -

Phase Transformations in Silica-Alumina- Magnesia Mixtures As Examined by Continuous X-Ray Diffraction: I
THE AMERICAN MINERALOGIST, VOL. 47, NOVEMBER-DECEMBER, 1962 PHASE TRANSFORMATIONS IN SILICA-ALUMINA- MAGNESIA MIXTURES AS EXAMINED BY CONTINUOUS X-RAY DIFFRACTION: I. TALC-KAOLINITE COMPOSITIONS R. B. Gnen,lF. M. Wnnr, aNp R. E. Gnru, Uniaersityof Illinois, (Jrbana, I llinois. Ansmacr The phase transformations of natural and synthetic oxide mixtures of talc-kaolinite compositlons were examined by continuous r-ray difiraction while heated to 1450' C. Dif- fraciion intensity data show the enhanced development of protoenstatite within the sheet structure of talc, thus indicating that the growth of this mineral on firing is largely con- trolled by the structure of the talc. The formation temperatule of cordierite, which was used as a measure of the reactivity of various starting materials, indicates that alumina in the form of mullite, rather than as corundum, is most efiective in lowering the develop- ment temperature of this mineral. Additional cordierite formation, through recrystalliza- tion on cooling indicates the presence of an intermediate licluid phase in the formation of this mineral. INrnotucrlow Generalsta,tement . Continuous high-temperature o-ray diffraction methods make it pos- sible to observehigh-temperature reactions as they occur (Wahl et aI., 1961). Previously, high-temperatrte n-ray diffraction has been used mainly for selected observations at some desired temperature and not for continuousobservations of a sampleas it is heated.The techniqueof continuous scanning was used in the present investigation for the study of high-temperaturereactions in talc, kaolinite, talc:kaolinite mixtures, and mixtures of oxides with compositions corresponding to talc and kaolinite. Infl,uence of starting materials Reactionrates between solids are known to be influencedby the nature of the starting materials. -

Spectral Reflectance and Chemical Properties of Magnesium-Rich Phyllosilicates
44th Lunar and Planetary Science Conference (2013) 2939.pdf SPECTRAL REFLECTANCE AND CHEMICAL PROPERTIES OF MAGNESIUM-RICH PHYLLOSILICATES. R.J. Léveillé1, E. Cloutis2, P. Mann2, P. Sobrón1, C. Lefebvre1, A. Koujelev1, 1Canadian Space Agency ([email protected]), 2University of Winnipeg, CANADA. Introduction: Identifying minerals present on Mars formed with a custom laboratory set-up at CSA. The is critical to reconstructing past environments and geo- set-up includes a Q-switch Nd:YAG laser generating 8 logical processes, and to assessing past habitability. ns pulses at 1064 nm (20mJ@ 1 Hz) and a spectrome- Various phyllosilicates have been identified in rocks on ter (Ocean Optics HR2000, 0.1nm resolution). An av- Mars, including at Gale Crater, by orbiter-based spec- erage spectrum was calculated based on 10 shots at 10 troscopy [e.g., 1-4]. The formation of these minerals closely spaced locations on pressed pellets. All ex- would have required the presence of persistent liquid periments were done in standard atmosphere, though water over extended periods of time. However, the we have also begun experiments under Mars-like at- timing of hydrologic activity and nature of mineral mospheric conditions. formation processes are poorly constrained [2]. While Al- and Fe-Mg-phyllosilicates seem to be most com- mon on Mars, Mg-rich phyllosilicates (e.g., serpentine, talc, saponite, Mg-smectite) have also been identified [3, 4]. Mg-rich phyllosilicates can be especially useful in identifying past geochemical conditions as they may preferentially indicate sustained aqueous conditions, either long-term presence of liquid water or high water- to-rock ratios. -

Read the Full Document
.." ..... ' ...,C(,- ,. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service (~ Food and Drug Administration \.:~f. College Park. MD 20740 APR 1 - 2014 Samuel S. Epstein, M.D. Cancer Prevention Coalition University of Illinois at Chicago School of Public Health, MC 922 2121 West Taylor Street, Rm. 322 Chicago, Illinois 60612 RE: Docket Numbers 94P-0420 and FDA-2008 -P-0309-000IlCP Dear Dr. Epstein: This letter is in response to your two Citizen Petitions dated November 17, 1994 and May 13 , 2008, requesting that the Food and Drug Administration (FDA or the Agency) require a cancer warning on cosmetic talc products. Your 1994 Petition requests that all cosmetic talc bear labels with a warning such as "Talcum powder causes cancer in laboratory animals. Frequent talc application in the female genital area increases the risk of ovarian cancer." Additionally, your 2008 Petition requests that cosmetic talcum powder products bear labels with a prominent warning such as: "Frequent talc application in the female genital area is responsible for major risks of ovarian cancer." Further, both of your Petitions specifically request, pursuant to 21 CFR 10.30(h)(2), a hearing for you to present scientific evidence in support of this petition. We have carefully considered both of your Petitions. We are committed to the protection of the public health and share your interest in reducing the risk of ovarian cancer. Current regulations state that cosmetic products shall bear a warning statement whenever necessary or appropriate to prevent a health hazard that may be associated with a product. FDA may publish a proposal to establish a regulation prescribing a warning statement on behalf of a petitioner if the petition is supported by adequate scientific basis on reasonable grounds. -
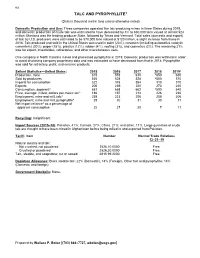
Talc Data Sheet
162 TALC AND PYROPHYLLITE1 (Data in thousand metric tons unless otherwise noted) Domestic Production and Use: Three companies operated five talc-producing mines in three States during 2019, and domestic production of crude talc was estimated to have decreased by 3% to 630,000 tons valued at almost $24 million. Montana was the leading producer State, followed by Texas and Vermont. Total sales (domestic and export) of talc by U.S. producers were estimated to be 570,000 tons valued at $120 million, a slight increase from those in 2018. Talc produced and sold in the United States was used in paint (23%), ceramics (including automotive catalytic converters) (20%), paper (15%), plastics (12%), rubber (4%), roofing (3%), and cosmetics (2%). The remaining 21% was for export, insecticides, refractories, and other miscellaneous uses. One company in North Carolina mined and processed pyrophyllite in 2019. Domestic production was withheld in order to avoid disclosing company proprietary data and was estimated to have decreased from that in 2018. Pyrophyllite was sold for refractory, paint, and ceramic products. Salient Statistics—United States: 2015 2016 2017 2018 2019e Production, mine 615 578 610 e650 630 Sold by producers 535 528 528 e550 570 Imports for consumption 322 378 354 313 310 Exports 206 239 220 273 240 Consumption, apparent2 651 668 662 e590 640 Price, average, milled, dollars per metric ton3 186 197 214 226 230 Employment, mine and mill, talc4 239 223 206 208 206 Employment, mine and mill, pyrophyllite4 29 30 31 30 31 Net import reliance5 as a percentage of apparent consumption 22 27 20 7 11 Recycling: Insignificant. -

Talc Resources of the Conterminous United States
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY TALC RESOURCES OF THE CONTERMINOUS UNITED STATES By Robert C. Greene J_/ Open-File Report OF 95-586 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. 1995 Menlo Park, CA 94025 Talc Resources of the conterminous United States Contents Abstract ....................................................................................4 Introduction ..............................................................................8 Talc and talc-bearing rocks .........................................................9 Physical, chemical, and optical properties of talc .........................10 Geology and Genesis of Talc Deposits ........................................11 Deposits in sedimentary rocks ..........................................11 Deposits in ultramafic rocks .............................................12 Uses for talc ............................................................................17 Mining and processing ..............................................................18 Environmental considerations ....................................................19 Acknowledgments ....................................................................19 California ...............................................................................20 Nevada ...................................................................................39 -
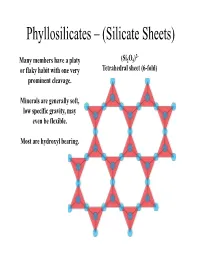
Phyllosilicates – (Silicate Sheets)
Phyllosilicates – (Silicate Sheets) 2- Many members have a platy (Si2O5) or flaky habit with one very Tetrahedral sheet (6-fold) prominent cleavage . Minerals are generally soft, low specific gravity, may even be flexible. Most are hydroxyl bearing. Each tetrahedra is bound to three neiggghboring tetrahedra via three basal bridging oxygens. The apical oxygen of each tetrahedral in a sheet all point in the same direction. The sheets are stacked either apice-to- apice or base-to-base. In an undistorted sheet the hydroxyl (OH) group sits in the centre and each outlined triangle is equivalent. Sheets within sheets…. Apical oxygens, plus the –OH group, coordinate a 6-fold (octahedral) site (XO6). These octahedral sites form infinitely extending sheets. All the octahedra lie on triangular faces, oblique to the tetrahedral sheets. The most common elements found in the 6 -fold site are Mg (or Fe) or Al . Dioctahedral vs Trioctahedral Mg and Al have different charges, but the sheet must remain charge neutral . With 6 coordinating oxygens, we have a partial charge of -6. How many Mg2+ ions are required to retain neutrality? How many Al3+ ions are required to retain neutrality? Mg occupies all octahedral sites, while Al will only occupy 2 out of every 3. The stacking of the sheets dictates the crystallography and c hem istry o f eac h o f t he p hhyll llosili cates. Trioctahedral Dioctahedral O Brucite Gibbsite Hydroxyl Magnesium Aluminium Trioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Phlogopite (Mg end-member biotite) Dioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Muscovite Compositional variation in phyllosilicates There is little solid solution between members of the dioctahedral and trioctahedral groups.