Avoid Common Pitfalls When Using Henry's
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modelling and Numerical Simulation of Phase Separation in Polymer Modified Bitumen by Phase- Field Method
http://www.diva-portal.org Postprint This is the accepted version of a paper published in Materials & design. This paper has been peer- reviewed but does not include the final publisher proof-corrections or journal pagination. Citation for the original published paper (version of record): Zhu, J., Lu, X., Balieu, R., Kringos, N. (2016) Modelling and numerical simulation of phase separation in polymer modified bitumen by phase- field method. Materials & design, 107: 322-332 http://dx.doi.org/10.1016/j.matdes.2016.06.041 Access to the published version may require subscription. N.B. When citing this work, cite the original published paper. Permanent link to this version: http://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-188830 ACCEPTED MANUSCRIPT Modelling and numerical simulation of phase separation in polymer modified bitumen by phase-field method Jiqing Zhu a,*, Xiaohu Lu b, Romain Balieu a, Niki Kringos a a Department of Civil and Architectural Engineering, KTH Royal Institute of Technology, Brinellvägen 23, SE-100 44 Stockholm, Sweden b Nynas AB, SE-149 82 Nynäshamn, Sweden * Corresponding author. Email: [email protected] (J. Zhu) Abstract In this paper, a phase-field model with viscoelastic effects is developed for polymer modified bitumen (PMB) with the aim to describe and predict the PMB storage stability and phase separation behaviour. The viscoelastic effects due to dynamic asymmetry between bitumen and polymer are represented in the model by introducing a composition-dependent mobility coefficient. A double-well potential for PMB system is proposed on the basis of the Flory-Huggins free energy of mixing, with some simplifying assumptions made to take into account the complex chemical composition of bitumen. -

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Chapter 2: Basic Tools of Analytical Chemistry
Chapter 2 Basic Tools of Analytical Chemistry Chapter Overview 2A Measurements in Analytical Chemistry 2B Concentration 2C Stoichiometric Calculations 2D Basic Equipment 2E Preparing Solutions 2F Spreadsheets and Computational Software 2G The Laboratory Notebook 2H Key Terms 2I Chapter Summary 2J Problems 2K Solutions to Practice Exercises In the chapters that follow we will explore many aspects of analytical chemistry. In the process we will consider important questions such as “How do we treat experimental data?”, “How do we ensure that our results are accurate?”, “How do we obtain a representative sample?”, and “How do we select an appropriate analytical technique?” Before we look more closely at these and other questions, we will first review some basic tools of importance to analytical chemists. 13 14 Analytical Chemistry 2.0 2A Measurements in Analytical Chemistry Analytical chemistry is a quantitative science. Whether determining the concentration of a species, evaluating an equilibrium constant, measuring a reaction rate, or drawing a correlation between a compound’s structure and its reactivity, analytical chemists engage in “measuring important chemical things.”1 In this section we briefly review the use of units and significant figures in analytical chemistry. 2A.1 Units of Measurement Some measurements, such as absorbance, A measurement usually consists of a unit and a number expressing the do not have units. Because the meaning of quantity of that unit. We may express the same physical measurement with a unitless number may be unclear, some authors include an artificial unit. It is not different units, which can create confusion. For example, the mass of a unusual to see the abbreviation AU, which sample weighing 1.5 g also may be written as 0.0033 lb or 0.053 oz. -

Solubility and Aggregation of Selected Proteins Interpreted on the Basis of Hydrophobicity Distribution
International Journal of Molecular Sciences Article Solubility and Aggregation of Selected Proteins Interpreted on the Basis of Hydrophobicity Distribution Magdalena Ptak-Kaczor 1,2, Mateusz Banach 1 , Katarzyna Stapor 3 , Piotr Fabian 3 , Leszek Konieczny 4 and Irena Roterman 1,2,* 1 Department of Bioinformatics and Telemedicine, Jagiellonian University—Medical College, Medyczna 7, 30-688 Kraków, Poland; [email protected] (M.P.-K.); [email protected] (M.B.) 2 Faculty of Physics, Astronomy and Applied Computer Science, Jagiellonian University, Łojasiewicza 11, 30-348 Kraków, Poland 3 Institute of Computer Science, Silesian University of Technology, Akademicka 16, 44-100 Gliwice, Poland; [email protected] (K.S.); [email protected] (P.F.) 4 Chair of Medical Biochemistry—Jagiellonian University—Medical College, Kopernika 7, 31-034 Kraków, Poland; [email protected] * Correspondence: [email protected] Abstract: Protein solubility is based on the compatibility of the specific protein surface with the polar aquatic environment. The exposure of polar residues to the protein surface promotes the protein’s solubility in the polar environment. The aquatic environment also influences the folding process by favoring the centralization of hydrophobic residues with the simultaneous exposure to polar residues. The degree of compatibility of the residue distribution, with the model of the concentration of hydrophobic residues in the center of the molecule, with the simultaneous exposure of polar residues is determined by the sequence of amino acids in the chain. The fuzzy oil drop model enables the quantification of the degree of compatibility of the hydrophobicity distribution Citation: Ptak-Kaczor, M.; Banach, M.; Stapor, K.; Fabian, P.; Konieczny, observed in the protein to a form fully consistent with the Gaussian 3D function, which expresses L.; Roterman, I. -
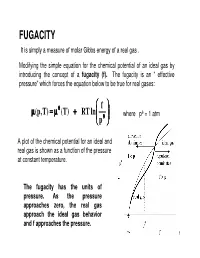
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

Solubility & Density
Natural Resources - Water WHAT’S SO SPECIAL ABOUT WATER: SOLUBILITY & DENSITY Activity Plan – Science Series ACTpa025 BACKGROUND Project Skills: Water is able to “dissolve” other substances. The molecules of water attract and • Discovery of chemical associate with the molecules of the substance that dissolves in it. Youth will have fun and physical properties discovering and using their critical thinking to determine why water can dissolve some of water. things and not others. Life Skills: • Critical thinking – Key vocabulary words: develops wider • Solvent is a substance that dissolves other substances, thus forming a solution. comprehension, has Water dissolves more substances than any other and is known as the “universal capacity to consider solvent.” more information • Density is a term to describe thickness of consistency or impenetrability. The scientific formula to determine density is mass divided by volume. Science Skills: • Hypothesis is an explanation for a set of facts that can be tested. (American • Making hypotheses Heritage Dictionary) • The atom is the smallest unit of matter that can take part in a chemical reaction. Academic Standards: It is the building block of matter. The activity complements • A molecule consists of two or more atoms chemically bonded together. this academic standard: • Science C.4.2. Use the WHAT TO DO science content being Activity: Density learned to ask questions, 1. Take a clear jar, add ¼ cup colored water, ¼ cup vegetable oil, and ¼ cup dark plan investigations, corn syrup slowly. Dark corn syrup is preferable to light so that it can easily be make observations, make distinguished from the oil. predictions and offer 2. -

THE SOLUBILITY of GASES in LIQUIDS Introductory Information C
THE SOLUBILITY OF GASES IN LIQUIDS Introductory Information C. L. Young, R. Battino, and H. L. Clever INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the literature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uniform format. The data for each system are evaluated and where data of sufficient accuracy are available values are recommended and in some cases a smoothing equation is given to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are published on consecutive pages. The following paper by E. Wilhelm gives a rigorous thermodynamic treatment on the solubility of gases in liquids. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas-liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solubility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the criti cal temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solubility depending on the particular range of pressure considered and the particular worker concerned. -

Chapter 15: Solutions
452-487_Ch15-866418 5/10/06 10:51 AM Page 452 CHAPTER 15 Solutions Chemistry 6.b, 6.c, 6.d, 6.e, 7.b I&E 1.a, 1.b, 1.c, 1.d, 1.j, 1.m What You’ll Learn ▲ You will describe and cate- gorize solutions. ▲ You will calculate concen- trations of solutions. ▲ You will analyze the colliga- tive properties of solutions. ▲ You will compare and con- trast heterogeneous mixtures. Why It’s Important The air you breathe, the fluids in your body, and some of the foods you ingest are solu- tions. Because solutions are so common, learning about their behavior is fundamental to understanding chemistry. Visit the Chemistry Web site at chemistrymc.com to find links about solutions. Though it isn’t apparent, there are at least three different solu- tions in this photo; the air, the lake in the foreground, and the steel used in the construction of the buildings are all solutions. 452 Chapter 15 452-487_Ch15-866418 5/10/06 10:52 AM Page 453 DISCOVERY LAB Solution Formation Chemistry 6.b, 7.b I&E 1.d he intermolecular forces among dissolving particles and the Tattractive forces between solute and solvent particles result in an overall energy change. Can this change be observed? Safety Precautions Dispose of solutions by flushing them down a drain with excess water. Procedure 1. Measure 10 g of ammonium chloride (NH4Cl) and place it in a Materials 100-mL beaker. balance 2. Add 30 mL of water to the NH4Cl, stirring with your stirring rod. -

Carbon Dioxide Capture by Chemical Absorption: a Solvent Comparison Study
Carbon Dioxide Capture by Chemical Absorption: A Solvent Comparison Study by Anusha Kothandaraman B. Chem. Eng. Institute of Chemical Technology, University of Mumbai, 2005 M.S. Chemical Engineering Practice Massachusetts Institute of Technology, 2006 SUBMITTED TO THE DEPARTMENT OF CHEMICAL ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN CHEMICAL ENGINEERING PRACTICE AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 2010 © 2010 Massachusetts Institute of Technology All rights reserved. Signature of Author……………………………………………………………………………………… Department of Chemical Engineering May 20, 2010 Certified by……………………………………………………….………………………………… Gregory J. McRae Hoyt C. Hottel Professor of Chemical Engineering Thesis Supervisor Accepted by……………………………………………………………………………….................... William M. Deen Carbon P. Dubbs Professor of Chemical Engineering Chairman, Committee for Graduate Students 1 2 Carbon Dioxide Capture by Chemical Absorption: A Solvent Comparison Study by Anusha Kothandaraman Submitted to the Department of Chemical Engineering on May 20, 2010 in partial fulfillment of the requirements of the Degree of Doctor of Philosophy in Chemical Engineering Practice Abstract In the light of increasing fears about climate change, greenhouse gas mitigation technologies have assumed growing importance. In the United States, energy related CO2 emissions accounted for 98% of the total emissions in 2007 with electricity generation accounting for 40% of the total1. Carbon capture and sequestration (CCS) is one of the options that can enable the utilization of fossil fuels with lower CO2 emissions. Of the different technologies for CO2 capture, capture of CO2 by chemical absorption is the technology that is closest to commercialization. While a number of different solvents for use in chemical absorption of CO2 have been proposed, a systematic comparison of performance of different solvents has not been performed and claims on the performance of different solvents vary widely. -

Selection of Thermodynamic Methods
P & I Design Ltd Process Instrumentation Consultancy & Design 2 Reed Street, Gladstone Industrial Estate, Thornaby, TS17 7AF, United Kingdom. Tel. +44 (0) 1642 617444 Fax. +44 (0) 1642 616447 Web Site: www.pidesign.co.uk PROCESS MODELLING SELECTION OF THERMODYNAMIC METHODS by John E. Edwards [email protected] MNL031B 10/08 PAGE 1 OF 38 Process Modelling Selection of Thermodynamic Methods Contents 1.0 Introduction 2.0 Thermodynamic Fundamentals 2.1 Thermodynamic Energies 2.2 Gibbs Phase Rule 2.3 Enthalpy 2.4 Thermodynamics of Real Processes 3.0 System Phases 3.1 Single Phase Gas 3.2 Liquid Phase 3.3 Vapour liquid equilibrium 4.0 Chemical Reactions 4.1 Reaction Chemistry 4.2 Reaction Chemistry Applied 5.0 Summary Appendices I Enthalpy Calculations in CHEMCAD II Thermodynamic Model Synopsis – Vapor Liquid Equilibrium III Thermodynamic Model Selection – Application Tables IV K Model – Henry’s Law Review V Inert Gases and Infinitely Dilute Solutions VI Post Combustion Carbon Capture Thermodynamics VII Thermodynamic Guidance Note VIII Prediction of Physical Properties Figures 1 Ideal Solution Txy Diagram 2 Enthalpy Isobar 3 Thermodynamic Phases 4 van der Waals Equation of State 5 Relative Volatility in VLE Diagram 6 Azeotrope γ Value in VLE Diagram 7 VLE Diagram and Convergence Effects 8 CHEMCAD K and H Values Wizard 9 Thermodynamic Model Decision Tree 10 K Value and Enthalpy Models Selection Basis PAGE 2 OF 38 MNL 031B Issued November 2008, Prepared by J.E.Edwards of P & I Design Ltd, Teesside, UK www.pidesign.co.uk Process Modelling Selection of Thermodynamic Methods References 1. -

THE SOLUBILITY of GASES in LIQUIDS INTRODUCTION the Solubility Data Project Aims to Make a Comprehensive Search of the Lit- Erat
THE SOLUBILITY OF GASES IN LIQUIDS R. Battino, H. L. Clever and C. L. Young INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the lit erature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uni form format. The data for each system are evaluated and where data of suf ficient accuracy are available values recommended and in some cases a smoothing equation suggested to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are pUblished on consecutive pages. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solUbility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solUbility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the critical temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solu bility depending on the particular range of pressure considered and the par ticular worker concerned. The difficulty partly stems from our inability to rigorously distinguish between a gas, a vapor, and a liquid, which has been discussed in numerous textbooks. -

Lecture 3. the Basic Properties of the Natural Atmosphere 1. Composition
Lecture 3. The basic properties of the natural atmosphere Objectives: 1. Composition of air. 2. Pressure. 3. Temperature. 4. Density. 5. Concentration. Mole. Mixing ratio. 6. Gas laws. 7. Dry air and moist air. Readings: Turco: p.11-27, 38-43, 366-367, 490-492; Brimblecombe: p. 1-5 1. Composition of air. The word atmosphere derives from the Greek atmo (vapor) and spherios (sphere). The Earth’s atmosphere is a mixture of gases that we call air. Air usually contains a number of small particles (atmospheric aerosols), clouds of condensed water, and ice cloud. NOTE : The atmosphere is a thin veil of gases; if our planet were the size of an apple, its atmosphere would be thick as the apple peel. Some 80% of the mass of the atmosphere is within 10 km of the surface of the Earth, which has a diameter of about 12,742 km. The Earth’s atmosphere as a mixture of gases is characterized by pressure, temperature, and density which vary with altitude (will be discussed in Lecture 4). The atmosphere below about 100 km is called Homosphere. This part of the atmosphere consists of uniform mixtures of gases as illustrated in Table 3.1. 1 Table 3.1. The composition of air. Gases Fraction of air Constant gases Nitrogen, N2 78.08% Oxygen, O2 20.95% Argon, Ar 0.93% Neon, Ne 0.0018% Helium, He 0.0005% Krypton, Kr 0.00011% Xenon, Xe 0.000009% Variable gases Water vapor, H2O 4.0% (maximum, in the tropics) 0.00001% (minimum, at the South Pole) Carbon dioxide, CO2 0.0365% (increasing ~0.4% per year) Methane, CH4 ~0.00018% (increases due to agriculture) Hydrogen, H2 ~0.00006% Nitrous oxide, N2O ~0.00003% Carbon monoxide, CO ~0.000009% Ozone, O3 ~0.000001% - 0.0004% Fluorocarbon 12, CF2Cl2 ~0.00000005% Other gases 1% Oxygen 21% Nitrogen 78% 2 • Some gases in Table 3.1 are called constant gases because the ratio of the number of molecules for each gas and the total number of molecules of air do not change substantially from time to time or place to place.