Rev 9/29/2015, 1 PROJECT PROTOCOL Quality in Prolapse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Obliterative Lefort Colpocleisis in a Large Group of Elderly Women
Obliterative LeFort Colpocleisis in a Large Group of Elderly Women Salomon Zebede, MD, Aimee L. Smith, MD, Leon N. Plowright, MD, Aparna Hegde, MD, Vivian C. Aguilar, MD, and G. Willy Davila, MD OBJECTIVE: To report on anatomical and functional satisfaction. Associated morbidity and mortality related outcomes, patient satisfaction, and associated morbidity to the procedure are low. Colpocleisis remains an and mortality in patients undergoing LeFort colpocleisis. excellent surgical option for the elderly patient with METHODS: This was a retrospective case series of advanced pelvic organ prolapse. LeFort colpocleisis performed from January 2000 to (Obstet Gynecol 2013;121:279–84) October 2011. Data obtained from a urogynecologic DOI: http://10.1097/AOG.0b013e31827d8fdb database included demographics, comorbidities, medi- LEVEL OF EVIDENCE: III cations, and urinary and bowel symptoms. Prolapse was quantified using the pelvic organ prolapse quantification y 2050, the elderly will represent the largest section (POP-Q) examination. Operative characteristics were Bof the population and pelvic floor dysfunction is recorded. All patients underwent pelvic examination projected to affect 58.2 million women in the United and POP-Q assessment at follow-up visits. Patients also States. We thus can expect to see a increase in the were asked about urinary and bowel symptoms as well as demand for urogynecologic services in this popula- overall satisfaction. All intraoperative and postoperative tion.1,2 Most women older than age 65 years are surgical complications were recorded. afflicted with at least one chronic medical condition, RESULTS: Three hundred twenty-five patients under- and, with the rate of comorbid conditions increasing went LeFort colpocleisis. -

Curriculum Vitae
CURRICULUM VITAE G. WILLY DAVILA, M.D. GUILLERMO H. DAVILA, M.D., FACOG, FPMRS Medical Director, Women and Children’s Services Holy Cross Medical Group Center for Urogynecology and Pelvic Floor Medicine Dorothy Mangurian Comprehensive Women’s Center Holy Cross Health Fort Lauderdale, Florida, USA Academic positions: Affiliate Professor, Florida Atlantic University School of Medicine Clinical Associate Professor, University of South Florida, Dept. of Obstetrics and Gynecology Address: Holy Cross HealthPlex Dorothy Mangurian Comprehensive Women’s Center 1000 NE 56th Street Fort Lauderdale, Florida 33334 Phone (954) 229-8660 FAX (954) 229-8659 Email: [email protected] Education 1976-1979 University of Texas at El Paso, Texas B.S. Biology 1976-1977 University of Bolivia Medical School, La Paz, Bolivia no degree 1979-1983 University of Texas Medical School, Houston, Texas M.D. Residency 1983-1987 University of Colorado Health Sciences Center, Denver, Colorado Obstetrics and Gynecology Postgraduate Training 1989 Gynecological Urology Clinical Preceptorship: Long Beach Memorial Hospital, University of California, Irvine Donald Ostergard, M.D., Director Previous Positions: 1999-2017 Chairman, Department of Gynecology, Cleveland Clinic Florida 1999-2018 Head, Section of Urogynecology and Reconstructive Pelvic Surgery Cleveland Clinic Florida Director, The Pelvic Floor Center at Cleveland Clinic Florida A National Association for Continence (NAFC) Center of Excellence in Pelvic Floor Care Director, Clinical Fellowship program - Urogynecology and Reconstructive Pelvic Surgery (2000-2007) 2015-2016 Clinical Director, Global Patient Services (Weston) Cleveland Clinic Foundation, International Center 2013-2016 Center Director, Obstetrics and Gynecology and Women’s Health Institute (Weston) Cleveland Clinic Foundation 1992-1999 Director, Colorado Gynecology and Continence Center, P.C. -

Colpocleisis (Closing the Vagina to Treat Prolapse)
Colpocleisis (Closing the vagina to treat prolapse) Patient Information Leaflet About this leaflet The information provided in this leaflet should be used as a guide. There may be some variation in how each gynaecologist performs the procedure, the care procedures on the ward immediately after your operation and the advice given to you when you get home. You should ask your gynaecologist about any concerns that you may have. You should take your time to read this leaflet. A page is provided at the end of the leaflet for you to write down any questions you may have. It is your right to know about your planned operation/procedure, why it has been recommended, what the alternatives are and what the risks and benefits are. These should be covered in this leaflet. You may also wish to ask about your gynaecologist’s personal experience and results of treating your condition. Benefits and risks The success and the risks of most operations carried out to treat prolapse and incontinence have been poorly studied and so it is often not possible to define them clearly. In this leaflet risks may be referred to as common, rare etc. or an approximate level of risk may be given. Further information about risk is explained in a leaflet published by the Royal College of Obstetricians and Gynaecologists “Understanding how risk is discussed in healthcare”. https://www.rcog.org.uk/globalassets/documents/patients/patient-information-leaflets/pi- understanding-risk.pdf The following table is taken from that leaflet British Society of Urogynaecology (BSUG) database In order to better understand the success and risks of surgery for prolapse and incontinence the British Society of Urogynaecology has established a national database. -
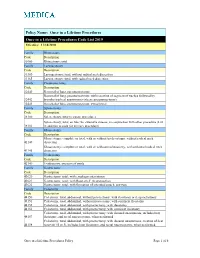
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Overview of Surgical Techniques in Gender-Affirming Genital Surgery
208 Review Article Overview of surgical techniques in gender-affirming genital surgery Mang L. Chen1, Polina Reyblat2, Melissa M. Poh2, Amanda C. Chi2 1GU Recon, Los Angeles, CA, USA; 2Southern California Permanente Medical Group, Los Angeles, CA, USA Contributions: (I) Conception and design: ML Chen, AC Chi; (II) Administrative support: None; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: None; (V) Data analysis and interpretation: None; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Amanda C. Chi. 6041 Cadillac Ave, Los Angeles, CA 90034, USA. Email: [email protected]. Abstract: Gender related genitourinary surgeries are vitally important in the management of gender dysphoria. Vaginoplasty, metoidioplasty, phalloplasty and their associated surgeries help patients achieve their main goal of aligning their body and mind. These surgeries warrant careful adherence to reconstructive surgical principles as many patients can require corrective surgeries from complications that arise. Peri- operative assessment, the surgical techniques employed for vaginoplasty, phalloplasty, metoidioplasty, and their associated procedures are described. The general reconstructive principles for managing complications including urethroplasty to correct urethral bulging, vaginl stenosis, clitoroplasty and labiaplasty after primary vaginoplasty, and urethroplasty for strictures and fistulas, neophallus and neoscrotal reconstruction after phalloplasty are outlined as well. Keywords: Transgender; vaginoplasty; phalloplasty; metoidioplasty Submitted May 30, 2019. Accepted for publication Jun 20, 2019. doi: 10.21037/tau.2019.06.19 View this article at: http://dx.doi.org/10.21037/tau.2019.06.19 Introduction the rectum and the lower urinary tract, formation of perineogenital complex for patients who desire a functional The rise in social awareness of gender dysphoria has led vaginal canal, labiaplasty, and clitoroplasty. -

Cervical Cancer
Cervical Cancer Ritu Salani, M.D., M.B.A. Assistant Professor, Dept. of Obstetrics & Gynecology Division of Gynecologic Oncology The Ohio State University Estimated gynecologic cancer cases United States 2010 Jemal, A. et al. CA Cancer J Clin 2010; 60:277-300 1 Estimated gynecologic cancer deaths United States 2010 Jemal, A. et al. CA Cancer J Clin 2010; 60:277-300 Decreasing Trends of Cervical Cancer Incidence in the U.S. • With the advent of the Pap smear, the incidence of cervical cancer has dramatically declined. • The curve has been stable for the past decade because we are not reaching the unscreened population. Reprinted by permission of the American Cancer Society, Inc. 2 Cancer incidence worldwide GLOBOCAN 2008 Cervical Cancer New cases Deaths United States 12,200 4,210 Developing nations 530,000 275,000 • 85% of cases occur in developing nations ¹Jemal, CA Cancer J Clin 2010 GLOBOCAN 2008 3 Cervical Cancer • Histology – Squamous cell carcinoma (80%) – Adenocarcinoma (15%) – Adenosquamous carcinoma (3 to 5%) – Neuroendocrine or small cell carcinoma (rare) Human Papillomavirus (HPV) • Etiologic agent of cervical cancer • HPV DNA sequences detected is more than 99% of invasive cervical carcinomas • High risk types: 16, 18, 45, and 56 • Intermediate types: 31, 33, 35, 39, 51, 52, 55, 58, 59, 66, 68 HPV 16 accounts for ~80% of cases HPV 18 accounts for 25% of cases Walboomers JM, Jacobs MV, Manos MM, et al. J Pathol 1999;189(1):12-9. 4 Viral persistence and Precancerous progression Normal lesion cervix Regression/ clearance Invasive cancer Risk factors • Early age of sexual activity • Cigggarette smoking • Infection by other microbial agents • Immunosuppression – Transplant medications – HIV infection • Oral contraceptive use • Dietary factors – Deficiencies in vitamin A and beta carotene 5 Multi-Stage Cervical Carcinogenesis Rosenthal AN, Ryan A, Al-Jehani RM, et al. -

OBGYN-Study-Guide-1.Pdf
OBSTETRICS PREGNANCY Physiology of Pregnancy: • CO input increases 30-50% (max 20-24 weeks) (mostly due to increase in stroke volume) • SVR anD arterial bp Decreases (likely due to increase in progesterone) o decrease in systolic blood pressure of 5 to 10 mm Hg and in diastolic blood pressure of 10 to 15 mm Hg that nadirs at week 24. • Increase tiDal volume 30-40% and total lung capacity decrease by 5% due to diaphragm • IncreaseD reD blooD cell mass • GI: nausea – due to elevations in estrogen, progesterone, hCG (resolve by 14-16 weeks) • Stomach – prolonged gastric emptying times and decreased GE sphincter tone à reflux • Kidneys increase in size anD ureters dilate during pregnancy à increaseD pyelonephritis • GFR increases by 50% in early pregnancy anD is maintaineD, RAAS increases = increase alDosterone, but no increaseD soDium bc GFR is also increaseD • RBC volume increases by 20-30%, plasma volume increases by 50% à decreased crit (dilutional anemia) • Labor can cause WBC to rise over 20 million • Pregnancy = hypercoagulable state (increase in fibrinogen anD factors VII-X); clotting and bleeding times do not change • Pregnancy = hyperestrogenic state • hCG double 48 hours during early pregnancy and reach peak at 10-12 weeks, decline to reach stead stage after week 15 • placenta produces hCG which maintains corpus luteum in early pregnancy • corpus luteum produces progesterone which maintains enDometrium • increaseD prolactin during pregnancy • elevation in T3 and T4, slight Decrease in TSH early on, but overall euthyroiD state • linea nigra, perineum, anD face skin (melasma) changes • increase carpal tunnel (median nerve compression) • increased caloric need 300cal/day during pregnancy and 500 during breastfeeding • shoulD gain 20-30 lb • increaseD caloric requirements: protein, iron, folate, calcium, other vitamins anD minerals Testing: In a patient with irregular menstrual cycles or unknown date of last menstruation, the last Date of intercourse shoulD be useD as the marker for repeating a urine pregnancy test. -

Gender Dysphoria Treatment – Community Plan Medical Policy
UnitedHealthcare® Community Plan Medical Policy Gender Dysphoria Treatment Policy Number: CS145.I Effective Date: March 1, 2021 Instructions for Use Table of Contents Page Related Community Plan Policies Application ..................................................................................... 1 • Blepharoplasty, Blepharoptosis, and Brow Ptosis Coverage Rationale ....................................................................... 1 Repair Definitions ...................................................................................... 3 • Botulinum Toxins A and B Applicable Codes .......................................................................... 4 • Cosmetic and Reconstructive Procedures Description of Services ................................................................. 8 • Gonadotropin Releasing Hormone Analogs Benefit Considerations .................................................................. 9 Clinical Evidence ........................................................................... 9 • Panniculectomy and Body Contouring Procedures U.S. Food and Drug Administration ........................................... 14 • Rhinoplasty and Other Nasal Surgeries References ................................................................................... 14 • Speech Language Pathology Services Policy History/Revision Information ........................................... 16 Commercial Policy Instructions for Use ..................................................................... 16 • Gender Dysphoria Treatment -

UNMH Obstetrics and Gynecology Clinical Privileges Name
UNMH Obstetrics and Gynecology Clinical Privileges Name:____________________________ Effective Dates: From __________ To ___________ All new applicants must meet the following requirements as approved by the UNMH Board of Trustees, effective April 28, 2017: Initial Privileges (initial appointment) Renewal of Privileges (reappointment) Expansion of Privileges (modification) INSTRUCTIONS: Applicant: Check off the “requested” box for each privilege requested. Applicants have the burden of producing information deemed adequate by the Hospital for a proper evaluation of current competence, current clinical activity, and other qualifications and for resolving any doubts related to qualifications for requested privileges. Department Chair: Check the appropriate box for recommendation on the last page of this form. If recommended with conditions or not recommended, provide condition or explanation. OTHER REQUIREMENTS: 1. Note that privileges granted may only be exercised at UNM Hospitals and clinics that have the appropriate equipment, license, beds, staff, and other support required to provide the services defined in this document. Site-specific services may be defined in hospital or department policy. 2. This document defines qualifications to exercise clinical privileges. The applicant must also adhere to any additional organizational, regulatory, or accreditation requirements that the organization is obligated to meet. --------------------------------------------------------------------------------------------------------------------------------------- -

OBGYN Outpatient Surgery Coding
OBGYN Outpatient Surgery Coding Anatomy Anatomy • Hyster/o – uterus, womb • Uter/o – uterus, womb • Metr/o – uterus, womb • Salping/o – tube, usually fallopian tube • Oophor/o – ovary • Ovari/o - ovary Terminology • Colpo – vagina • Cervic/o – cervix, lower part of the uterus, the “neck” • Episi/o – vulva • Vulv/o – vulva • Perine/o – the space between the anus and vulva Hysterectomy • A hysterectomy is an operation to remove a woman's uterus. • A woman may have a hysterectomy for different reasons, including: • Uterine fibroids that cause pain • bleeding, or other problems. • Uterine prolapse, which is a sliding of the uterus from its normal position into the vaginal canal. Hysterectomy • There are around 30 hysterectomy CPT codes. • To find the correct code you have to first check: • the surgical approach and • extent of the procedure. Surgical Approaches • Abdominal – the uterus is removed via an incision in the lower abdomen • Vaginal – the uterus is removed via an incision in the vagina • Laparoscopic – the procedure is performed using a laparoscope , inserted via several small incisions in the body. • Their are also CPT codes for laparoscopic-assisted vaginal approach. In this procedure ,the scope is inserted via a small incisions in the vagina. Extent of Procedure • Total hysterectomy: It includes laparoscopically detaching the entire uterine cervix and body from the surrounding supporting structures and suturing the vaginal cuff. It includes bivalving, coring, or morcellating the excised tissues, as required. The uterus is then removed through the vagina or abdomen. • Subtotal, partial or supracervical hysterectomy: It is the removal of the fundus or op portion of the uterus only, leaving the cervix in place. -

SJH Procedures
SJH Procedures - Gynecology and Gynecology Oncology Services New Name Old Name CPT Code Service ABLATION, LESION, CERVIX AND VULVA, USING CO2 LASER LASER VAPORIZATION CERVIX/VULVA W CO2 LASER 56501 Destruction of lesion(s), vulva; simple (eg, laser surgery, Gynecology electrosurgery, cryosurgery, chemosurgery) 56515 Destruction of lesion(s), vulva; extensive (eg, laser surgery, Gynecology electrosurgery, cryosurgery, chemosurgery) 57513 Cautery of cervix; laser ablation Gynecology BIOPSY OR EXCISION, LESION, FACE AND NECK EXCISION/BIOPSY (MASS/LESION/LIPOMA/CYST) FACE/NECK General, Gynecology, Plastics, ENT, Maxillofacial BIOPSY OR EXCISION, LESION, FACE AND NECK, 2 OR MORE EXCISE/BIOPSY (MASS/LESION/LIPOMA/CYST) MULTIPLE FACE/NECK 11102 Tangential biopsy of skin (eg, shave, scoop, saucerize, curette); General, Gynecology, single lesion Aesthetics, Urology, Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11103 Tangential biopsy of skin (eg, shave, scoop, saucerize, curette); General, Gynecology, each separate/additional lesion (list separately in addition to Aesthetics, Urology, code for primary procedure) Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11104 Punch biopsy of skin (including simple closure, when General, Gynecology, performed); single lesion Aesthetics, Urology, Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11105 Punch biopsy of skin (including simple closure, when General, Gynecology, performed); each separate/additional lesion -

Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes
UnitedHealthcare® Commercial Policy Appendix: Applicable Code List Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes This list of codes applies to the Utilization Review Guideline titled Effective Date: August 1, 2021 Outpatient Surgical Procedures – Site of Service. Applicable Codes The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. The listing of a code does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply. This list contains CPT/HCPCS codes for the following: • Auditory System • Female Genital System • Musculoskeletal System • Cardiovascular System • Hemic and Lymphatic Systems • Nervous System • Digestive System • Integumentary System • Respiratory System • Eye/Ocular Adnexa System • Male Genital System • Urinary System CPT Code Description Auditory System 69100 Biopsy external ear 69110 Excision external ear; partial, simple repair 69140 Excision exostosis(es), external auditory canal 69145 Excision soft tissue lesion, external auditory canal 69205 Removal foreign body from external auditory canal; with general anesthesia 69222 Debridement, mastoidectomy cavity, complex (e.g., with anesthesia or more