CREOG Review: Oncology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gonadal (Testicular and Ovarian Tumors)
Gonadal (Testicular and Ovarian Tumors) Gonadal (Testicular and Ovarian Tumors) Authors: Ayda G. Nambayan, DSN, RN, St. Jude Children’s Research Hospital Erin Gafford, Pediatric Oncology Education Student, St. Jude Children’s Research Hospital; Nursing Student, School of Nursing, Union University Content Reviewed by: Guillermo L. Chantada, MD, Hospital JP Garrahan, Buenos Aires, Argentina Cure4Kids Release Date: 1 September 2006 At 4 weeks gestation, germ cells begin to develop in the yolk sac in an undifferentiated state. These primordial germ cells migrate to the gonadal ridge by the 6th week of gestation, and descend into the pelvis or scrotal sac.This migratory route explains the midline location of most extra-gonadal germ cell tumors (intracranial, mediastinal, retroperitoneal or sacrococcygeal). (A -1 ) Germ cell neoplasms arise either from the primordial germ cells (gonadal) or indirectly through embryonic or extra-embryonic differentiation (extra-gonadal). The stromal tumors arise from the primitive sex cords of either the ovary or the testicles, and the rare epithelial tumors arise from the coelomic epithelium that have undergone neoplastic transformations. Though the morphology of each type of germ cell tumor is similar in all locations (whether gonadal or extragonadal), the morphologic type and biological characteristics vary depending on the site of origin and age of the patient. There are three (A – 2) biologically distinct subsets of germ cell tumors: - tumors of the adolescent testis and ovary - extragonadal germ cell tumors of older children - tumors of infants and young children Testicular Germ Cell Tumors: Approximately 7% of all germ cell tumors are testicular and 75% of all (A – 3) testicular tumors have a germ cell origin. -

Ovarian Cancer and Cervical Cancer
What Every Woman Should Know About Gynecologic Cancer R. Kevin Reynolds, MD The George W. Morley Professor & Chief, Division of Gyn Oncology University of Michigan Ann Arbor, MI What is gynecologic cancer? Cancer is a disease where cells grow and spread without control. Gynecologic cancers begin in the female reproductive organs. The most common gynecologic cancers are endometrial cancer, ovarian cancer and cervical cancer. Less common gynecologic cancers involve vulva, Fallopian tube, uterine wall (sarcoma), vagina, and placenta (pregnancy tissue: molar pregnancy). Ovary Uterus Endometrium Cervix Vagina Vulva What causes endometrial cancer? Endometrial cancer is the most common gynecologic cancer: one out of every 40 women will develop endometrial cancer. It is caused by too much estrogen, a hormone normally present in women. The most common cause of the excess estrogen is being overweight: fat cells actually produce estrogen. Another cause of excess estrogen is medication such as tamoxifen (often prescribed for breast cancer treatment) or some forms of prescribed estrogen hormone therapy (unopposed estrogen). How is endometrial cancer detected? Almost all endometrial cancer is detected when a woman notices vaginal bleeding after her menopause or irregular bleeding before her menopause. If bleeding occurs, a woman should contact her doctor so that appropriate testing can be performed. This usually includes an endometrial biopsy, a brief, slightly crampy test, performed in the office. Fortunately, most endometrial cancers are detected before spread to other parts of the body occurs Is endometrial cancer treatable? Yes! Most women with endometrial cancer will undergo surgery including hysterectomy (removal of the uterus) in addition to removal of ovaries and lymph nodes. -

Vaginal and Vulvar Cancer 10.1136/Ijgc-2020-ESGO.178
Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-ESGO.177 on 4 December 2020. Downloaded from Abstracts 520 LONG TERM FOLLOW UP AFTER DIAGNOSIS OF Introduction/Background Since the introduction of the S2K GESTATIONAL TROPHOBLASTIC DISEASE AWMF guideline-based sentinel node biopsy technique in uni- focal vulvar cancer (diameter of <4 cm) and unsuspicious Pedro Corvelo Freitas, Beatriz Mira, António Guimarães, Ana Opinião, Hugo Nunes, Ana Francisca Jorge, Fátima Vaz, António Moreira. Instituto Português de Oncologia de Lisboa groin lymph nodes, the morbidity rate of patients has signifi- Francisco Gentil cantly decreased in Germany. The groin recurrence rate after IFL is vary from 0% to 5.8%, in contrast to 2.3% (95% CI, 10.1136/ijgc-2020-ESGO.176 0.6% to 5%) in unifocal vulvar cancer vs 3% (95% CI, 1% to 6%) in multifocal vulvar cancer after SLNB only, as sug- Introduction/Background The spectrum of Gestational tropho- gested in the GRoningen INternational Study on Sentinel blastic disease (GTD) ranges from pre-malignant conditions of node in Vulvar cancer (GROINSS-V-I) in 2008. Current guide- complete (CHM) and partial (PHM) hydatidiform moles to the lines suggest that in cases of metastasis of unilateral sentinel malignant invasive mole, choriocarcinoma (CC) and very rare lymph node (SLN) biopsy (B), groin node dissection, namely placental site trophoblastic tumour/epithelioid trophoblastic inguinofemoral lymphadenectomy (IFL), should be performed tumour (PSTT/ETT). Gestational trophoblastic neoplasia (GTN) bilaterally. However, a publication by Woelber et al. in Ger- are highly responsive to chemotherapy (CT) and with appropri- many and and Nica et al. -

Primary Immature Teratoma of the Thigh Fig
CORRESPONDENCE 755 8. Gray W, Kocjan G. Diagnostic Cytopathology. 2nd ed. London: Delete all that do not apply: Elsevier Health Sciences, 2003; 677. 9. Richards A, Dalrymple C. Abnormal cervicovaginal cytology, unsatis- Cervix, colposcopic biopsy/LLETZ/cone biopsy: factory colposcopy and the use of vaginal estrogen cream: an obser- vational study of clinical outcomes for women in low estrogen states. Diagnosis: NIL (No intraepithelial lesion WHO 2014) J Obstet Gynaecol Res 2015; 41: 440e4. LSIL (CIN 1 with HPV effect WHO 2014) 10. Darragh TM, Colgan TJ, Cox T, et al. The lower anogenital squamous HSIL (CIN2/3 WHO 2014) terminology standardization project for HPV-associated lesions: back- Squamous cell carcinoma ground and consensus recommendation from the College of American Immature squamous metaplasia Pathologists and the American Society for Colposcopy and Cervical Adenocarcinoma in situ (AIS, HGGA) e Adenocarcinoma Pathology. Arch Pathol Lab Med 2012; 136: 1267 97. Atrophic change 11. McCluggage WG. Endocervical glandular lesions: controversial aspects e Extending into crypts: Not / Idenfied and ancillary techniques. J Clin Pathol 2013; 56: 164 73. Epithelial stripping: Not / Present 12. World Health Organization (WHO). Comprehensive Cervical Cancer Invasive disease: Not / Idenfied / Micro-invasive Control: A Guide to Essential Practice. 2nd ed. Geneva: WHO, 2014. Depth of invasion: mm Transformaon zone: Not / Represented Margins: DOI: https://doi.org/10.1016/j.pathol.2019.07.014 Ectocervical: Not / Clear Endocervical: Not / Clear Circumferenal: Not / Clear p16 status: Negave / Posive Primary immature teratoma of the thigh Fig. 3 A proposed synoptic reporting format for pathologists reporting colposcopic biopsies and cone biopsies or LLETZ. Sir, Teratomas are germ cell tumours composed of a variety of HSIL, AIS, micro-invasive or more advanced invasive dis- somatic tissues derived from more than one germ layer 12 ease. -

Squamous Cell Carcinoma Arising in an Ovarian Mature Cystic Teratoma
Case Report Obstet Gynecol Sci 2013;56(2):121-125 http://dx.doi.org/10.5468/OGS.2013.56.2.121 pISSN 2287-8572 · eISSN 2287-8580 Squamous cell carcinoma arising in an ovarian mature cystic teratoma complicating pregnancy Nae-Ri Yun1, Jung-Woo Park1, Min-Kyung Hyun1, Jee-Hyun Park1, Suk-Jin Choi2, Eunseop Song1 Departments of 1Obstetrics and Gynecology and 2Pathology, Inha University College of Medicine, Incheon, Korea Mature cystic teratomas of the ovary (MCT) are usually observed in women of reproductive age with the most dreadful complication being malignant transformation which occurs in approximately 1% to 3% of MCTs. In this case report, we present a patient with squamous cell carcinoma which developed from a MCT during pregnancy. The patient was treated conservatively without adjuvant chemotherapy and was followed without evidence of disease for more than 60 months using conventional tools as well as positron emission tomography-computed tomography following the initial surgery. We report this case along with the review of literature. Keywords: Dermoid cyst; Malignant transformation; Observation; Positron emission tomography-computed tomography Introduction An 18 cm solid and cystic left ovarian mass with a smooth surface and two small right ovarian cysts were detected re- The incidence of adnexal masses during pregnancy is 1% to sulting in a laparotomy at 13 weeks of gestation and left sal- 9% [1]. Mature cystic teratomas (MCT) are common during pingo-oophorectomy and right ovarian cystectomy (Fig. 1C). pregnancy with the most dreadful complication being ma- The report of the frozen section from both tissues revealed lignant transformation which occurs in approximately 1% to MCT. -

Pembrolizumab in Vaginal and Vulvar Squamous Cell Carcinoma: a Case Series from a Phase II Basket Trial Jefrey A
www.nature.com/scientificreports OPEN Pembrolizumab in vaginal and vulvar squamous cell carcinoma: a case series from a phase II basket trial Jefrey A. How 1, Amir A. Jazaeri 1, Pamela T. Soliman1, Nicole D. Fleming1, Jing Gong2, Sarina A. Piha‑Paul2, Filip Janku 2, Bettzy Stephen 2 & Aung Naing 2* Vaginal and vulvar squamous cell carcinoma (SCC) are rare tumors that can be challenging to treat in the recurrent or metastatic setting. We present a case series of patients with vaginal or vulvar SCC who were treated with single‑agent pembrolizumab as part of a phase II basket clinical trial to evaluate efcacy and safety. Two cases of recurrent and metastatic vaginal SCC, with multiple prior lines of systemic chemotherapy and radiation, received pembrolizumab. One patient had signifcant reduction (81%) in target tumor lesions prior to treatment discontinuation at cycle 10 following confrmed progression of disease with new metastatic lesions (stable disease by irRECIST criteria). In contrast, the other patient with vaginal SCC discontinued treatment after cycle 3 due to disease progression. Both patients had PD‑L1 positive vaginal tumors and tolerated treatment well. One case of recurrent vulvar SCC with multiple surgical resections and prior progression on systemic carboplatin had a 30% reduction in her target tumor lesions following pembrolizumab treatment with a PD‑L1 positive tumor. Treatment was discontinued for grade 3 mucositis after cycle 5. Pembrolizumab may provide some clinical beneft to some patients with vaginal or vulvar SCC and is overall safe to utilize in this population. Future studies are needed to evaluate the efcacy of pembrolizumab in these rare tumor types and to identify predictive biomarkers of response. -

Sexual Dysfunction in Gynecologic Cancer Patients
WCRJ 2017; 4 (1): e835 SEXUAL DYSFUNCTION IN GYNECOLOGIC CANCER PATIENTS L. DEL PUP Division of Gynecological Oncology, CRO Aviano, National Cancer Institute, Aviano (PN), Italy. Abstract – Objective: Sexual dysfunction is prevalent among gynecologic cancer survivors and strongly impacts on the quality of life (QoL), but the subject is poorly diagnosed and treated. Materials and Methods: A comprehensive literature search of English language studies on sex- ual dysfunctions due to gynecologic cancer treatment has been conducted on MEDLINE databases. Results: Surgery, radiation, and chemotherapy can cause any kind of sexual dysfunction with different mechanisms: psychological and relational, hormonal and pharmacological, neurological and vascular, side effects of chemo and radiation therapies, and direct effects of surgery on sexually involved pelvic organs. Many patients expect their healthcare providers to address sexual health concerns, but most have never discussed sex-related issues with their physician, or they do not re- ceive a proper treatment or referral. This can have medical legal consequences, because it must be discussed and documented before starting treatment. Conclusions: Oncology providers can make a significant impact on the QoL of gynecologic cancer sur- vivors by informing patients and by asking them for sexual health concerns. Counseling is per se beneficial, as it improves QoL. Furthermore, it permits a proper referral and resolution of most symptoms. KEYWORDS: Gynecologic cancer, Sexual dysfunction, Vulvar cancer, Vaginal cancer, Cervical cancer, Endometrial cancer, Ovarian cancer, Ospemifene. INTRODUCTION ly ask about sexual issues, but 64% state that phy- sicians never initiates the conversation during their The proportion of people living with and surviv- care3. ing gynecologic cancer is growing. -

Endometrial Cancer As a Metabolic Disease with Dysregulated PI3K Signaling: Shedding Light on Novel Therapeutic Strategies
International Journal of Molecular Sciences Review Endometrial Cancer as a Metabolic Disease with Dysregulated PI3K Signaling: Shedding Light on Novel Therapeutic Strategies Satoru Kyo * and Kentaro Nakayama Department of Obstetrics and Gynecology, Shimane University Faculty of Medicine, 89-1 Enya-cho, Izumo, Shimane 693-8501, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-(0)853-20-2268; Fax: +81-(0)853-20-2264 Received: 3 July 2020; Accepted: 21 August 2020; Published: 23 August 2020 Abstract: Endometrial cancer (EC) is one of the most common malignancies of the female reproductive organs. The most characteristic feature of EC is the frequent association with metabolic disorders. However, the components of these disorders that are involved in carcinogenesis remain unclear. Accumulating epidemiological studies have clearly revealed that hyperinsulinemia, which accompanies these disorders, plays central roles in the development of EC via the insulin-phosphoinositide 3 kinase (PI3K) signaling pathway as a metabolic driver. Recent comprehensive genomic analyses showed that over 90% of ECs have genomic alterations in this pathway, resulting in enhanced insulin signaling and production of optimal tumor microenvironments (TMEs). Targeting PI3K signaling is therefore an attractive treatment strategy. Several clinical trials for recurrent or advanced ECs have been attempted using PI3K-serine/threonine kinase (AKT) inhibitors. However, these agents exhibited far lower efficacy than expected, possibly due to activation of alternative pathways that compensate for the PIK3-AKT pathway and allow tumor growth, or due to adaptive mechanisms including the insulin feedback pathway that limits the efficacy of agents. Overcoming these responses with careful management of insulin levels is key to successful treatment. -

Papillary Carcinoma in Mature Teratoma of Struma Ovarii
Srbovan, D et al 2015 Papillary Carcinoma in Mature Teratoma of Struma Ovarii. Journal of the Belgian Society of Radiology, 99(1), pp. 76–78, DOI: http://dx.doi.org/10.5334/jbr-btr.855 CASE REPORT Papillary Carcinoma in Mature Teratoma of Struma Ovarii D. Srbovan*, J. Mihailović*, K. Nikoletić*, E. Matovina† and N. Šolajić† A 62-year-old woman had the incidental finding of malignant struma ovarii following surgery for primary endometrial carcinoma. The patient had vaginal bleeding for one year. After gynecological examination, she was referred for fractional curettage which revealed endometrial cancer. The patient underwent total hysterectomy and bilateral adnexectomy. Histological findings of uterus confirm the presence of endo- metrial cancer. The left ovary showed the presence of mature teratoma with dominant thyroid tissue and focus of papillary carcinoma. Postoperatively she underwent radiation therapy and 3 months later total thyroidectomy. The stimulated thyroglobulin level was detectable. She was referred for radioiodine abla- tion with a dose of 3,7GBq 131-J. Post therapy scintigraphy shows pathological uptake of 131-J only in the neck. The patient continued treatment of endometrial cancer (external beam therapy). She is cur- rently on suppressive hormone L-thyroxin therapy. Two months later hormonal status, thyroglobulin and antithyroglobulin antibodies showed optimal range. Keyword: Teratoma Struma ovarii is a rare form of germ cell derived ovarian Case report tumor defined by the presence of thyroid tissue compris- A 62-year-old woman was admitted to the Department of ing more than 50% of the overall mass [1]. Struma ovarii Gynecology at the Institute of Oncology Vojvodina with was first described in 1899 which literally means “goiter of the complaint of vaginal bleeding for one year. -
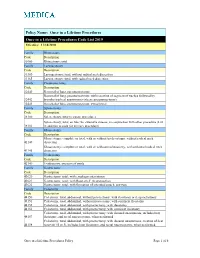
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Incidence and Cost of Anal, Penile, Vaginal and Vulvar Cancer in Denmark Jens Olsen1*, Tine Rikke Jørgensen2, Kristian Kofoed3 and Helle Kiellberg Larsen3
Olsen et al. BMC Public Health 2012, 12:1082 http://www.biomedcentral.com/1471-2458/12/1082 RESEARCH ARTICLE Open Access Incidence and cost of anal, penile, vaginal and vulvar cancer in Denmark Jens Olsen1*, Tine Rikke Jørgensen2, Kristian Kofoed3 and Helle Kiellberg Larsen3 Abstract Background: Besides being a causative agent for genital warts and cervical cancer, human papillomavirus (HPV) contributes to 40-85% of cases of anal, penile, vaginal and vulvar cancer and precancerous lesions. HPV types 16 & 18 in particular contribute to 74-93% of these cases. Overall the number of new cases of these four cancers may be relatively high implying notable health care cost to society. The aim of this study was to estimate the incidence and the health care sector costs of anal, penile, vaginal and vulvar cancer. Methods: New anogenital cancer patients were identified from the Danish National Cancer Register using ICD-10 diagnosis codes. Resource use in the health care sector was estimated for the year prior to diagnosis, and for the first, second and third years after diagnosis. Hospital resource use was defined in terms of registered hospital contacts, using DRG (Diagnosis Related Groups) and DAGS (Danish Outpatient Groups System) charges as cost estimates for inpatient and outpatient contacts, respectively. Health care consumption by cancer patients diagnosed in 2004–2007 was compared with that by an age- and sex-matched cohort without cancer. Hospital costs attributable to four anogenital cancers were estimated using regression analysis. Results: The annual incidence of anal cancer in Denmark is 1.9 per 100,000 persons. The corresponding incidence rates for penile, vaginal and vulvar cancer are 1.7, 0.9 and 3.6 per 100,000 males/females, respectively. -

Overview of Surgical Techniques in Gender-Affirming Genital Surgery
208 Review Article Overview of surgical techniques in gender-affirming genital surgery Mang L. Chen1, Polina Reyblat2, Melissa M. Poh2, Amanda C. Chi2 1GU Recon, Los Angeles, CA, USA; 2Southern California Permanente Medical Group, Los Angeles, CA, USA Contributions: (I) Conception and design: ML Chen, AC Chi; (II) Administrative support: None; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: None; (V) Data analysis and interpretation: None; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Amanda C. Chi. 6041 Cadillac Ave, Los Angeles, CA 90034, USA. Email: [email protected]. Abstract: Gender related genitourinary surgeries are vitally important in the management of gender dysphoria. Vaginoplasty, metoidioplasty, phalloplasty and their associated surgeries help patients achieve their main goal of aligning their body and mind. These surgeries warrant careful adherence to reconstructive surgical principles as many patients can require corrective surgeries from complications that arise. Peri- operative assessment, the surgical techniques employed for vaginoplasty, phalloplasty, metoidioplasty, and their associated procedures are described. The general reconstructive principles for managing complications including urethroplasty to correct urethral bulging, vaginl stenosis, clitoroplasty and labiaplasty after primary vaginoplasty, and urethroplasty for strictures and fistulas, neophallus and neoscrotal reconstruction after phalloplasty are outlined as well. Keywords: Transgender; vaginoplasty; phalloplasty; metoidioplasty Submitted May 30, 2019. Accepted for publication Jun 20, 2019. doi: 10.21037/tau.2019.06.19 View this article at: http://dx.doi.org/10.21037/tau.2019.06.19 Introduction the rectum and the lower urinary tract, formation of perineogenital complex for patients who desire a functional The rise in social awareness of gender dysphoria has led vaginal canal, labiaplasty, and clitoroplasty.