A Morphometric Analysis of Trimeres Ur Us Vo Geli (David, Vidal and Pauwels, 2001), with New Data on Diagnostic Characteristics, Distribution and Natural History
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On Further Specimens of the Pit Viper Trimeresurus Erythrurus
Journal of Animal Diversity Online ISSN 2676-685X Volume 3, Issue 1 (2021) Research Article http://dx.doi.org/10.52547/JAD.2021.3.1.7 On further specimens of the Pit viper Trimeresurus erythrurus (Cantor, 1839) (Squamata: Viperidae), with description of a topotype and range extension to the Godavari Basin, peninsular India Kaushik Deutiˡ, Ramaswamy Aengals², Sujoy Rahaˡ, Sudipta Debnathˡ, Ponnusamy Sathiyaselvam3 and Sumaithangi Rajagopalan Ganesh4* 1Zoological Survey of India, Herpetology Division, 27 JL Nehru Road, Kolkata 700016, West Bengal, India ²Zoological Survey of India, Sunderbans Field Research Center, Canning 743329, West Bengal, India 3Bombay Natural History Society, Hornbill House, Shaheed Bhagat Singh Marg, Mumbai 400023, India 4Chennai Snake Park, Rajbhavan post, Chennai 600022, Tamil Nadu, India *Corresponding author : [email protected] Abstract We report on a topotypical specimen of the spot-tailed pit viper Trimeresurus erythrurus recorded from Sunderbans in India and a distant, southerly, range extension from Kakinada mangroves, based on preserved (n= 1, seen in 2019) and live uncollected (n= 2; seen in 2014) specimens, respectively. The specimens Received: 26 December 2020 (n= 3) share the following characteristics: verdant green dorsum, yellow iris, Accepted: 27 January 2021 white ventrolateral stripes in males, 23 midbody scale rows, 161–172 ventrals, Published online: 17 July 2021 61–76 subcaudals, and reddish tail tip. Drawing on the published records, its apparent rarity within its type locality and lack of records from the Circar Coast of India, our study significantly adds to the knowledge of the distribution and morphology of this species. Being a medically important venomous snake, its presence in the Godavari mangrove basin calls for wider dissemination of this information among medical practitioners, in addition to fundamental researchers like academics and herpetologists. -

New Records of Snakes (Squamata: Serpentes) from Hoa Binh Province, Northwestern Vietnam
Bonn zoological Bulletin 67 (1): 15–24 May 2018 New records of snakes (Squamata: Serpentes) from Hoa Binh Province, northwestern Vietnam Truong Quang Nguyen1,2,*, Tan Van Nguyen 1,3, Cuong The Pham1,2, An Vinh Ong4 & Thomas Ziegler5 1 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam 2 Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam 3 Save Vietnam’s Wildlife, Cuc Phuong National Park, Ninh Binh Province, Vietnam 4 Vinh University, 182 Le Duan Road, Vinh City, Nghe An Province, Vietnam 5 AG Zoologischer Garten Köln, Riehler Strasse 173, D-50735 Cologne, Germany * Corresponding author. E-mail: [email protected] Abstract. We report nine new records of snakes from Hoa Binh Province based on a reptile collection from Thuong Tien, Hang Kia-Pa Co, Ngoc Son-Ngo Luong nature reserves, and Tan Lac District, comprising six species of Colubri- dae (Dryocalamus davisonii, Euprepiophis mandarinus, Lycodon futsingensis, L. meridionalis, Sibynophis collaris and Sinonatrix aequifasciata), one species of Pareatidae (Pareas hamptoni) and two species of Viperidae (Protobothrops mu- crosquamatus and Trimeresurus gumprechti). In addition, we provide an updated list of 43 snake species from Hoa Binh Province. The snake fauna of Hoa Binh contains some species of conservation concern with seven species listed in the Governmental Decree No. 32/2006/ND-CP (2006), nine species listed in the Vietnam Red Data Book (2007), and three species listed in the IUCN Red List (2018). Key words. New records, snakes, taxonomy, Hoa Binh Province. -

On Trimeresurus Sumatranus
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/266262458 On Trimeresurus sumatranus (Raffles, 1822), with the designation of a neotype and the description of a new species of pitviper from Sumatra (Squamata: Viperidae: Crotalinae) Article in Amphibian and Reptile Conservation · September 2014 CITATIONS READS 4 360 3 authors, including: Gernot Vogel Irvan Sidik Independent Researcher Indonesian Institute of Sciences 102 PUBLICATIONS 1,139 CITATIONS 12 PUBLICATIONS 15 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Save Vietnam Biodiversity View project Systematics of the genus Pareas View project All content following this page was uploaded by Gernot Vogel on 01 October 2014. The user has requested enhancement of the downloaded file. Comparative dorsal view of the head of Trimeresurus gunaleni spec. nov. (left) and T. sumatranus (right). Left from above: male, female (holotype), male, all alive, from Sumatra Utara Province, Sumatra. Right: adult female alive from Bengkulu Province, Su- matra, adult male alive from Bengkulu Province, Sumatra, preserved female from Borneo. Photos: N. Maury. Amphib. Reptile Conserv. | amphibian-reptile-conservation.org (1) September 2014 | Volume 8 | Number 2 | e80 Copyright: © 2014 Vogel et al. This is an open-access article distributed under the terms of the Creative Commons Attribution–NonCommercial–NoDerivs 3.0 Unported License, Amphibian & Reptile Conservation which permits -

Venomics of Trimeresurus (Popeia) Nebularis, the Cameron Highlands Pit Viper from Malaysia: Insights Into Venom Proteome, Toxicity and Neutralization of Antivenom
toxins Article Venomics of Trimeresurus (Popeia) nebularis, the Cameron Highlands Pit Viper from Malaysia: Insights into Venom Proteome, Toxicity and Neutralization of Antivenom Choo Hock Tan 1,*, Kae Yi Tan 2 , Tzu Shan Ng 2, Evan S.H. Quah 3 , Ahmad Khaldun Ismail 4 , Sumana Khomvilai 5, Visith Sitprija 5 and Nget Hong Tan 2 1 Department of Pharmacology, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia; 2 Department of Molecular Medicine, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] (K.Y.T.); [email protected] (T.S.N.); [email protected] (N.H.T.) 3 School of Biological Sciences, Universiti Sains Malaysia, 11800 Minden, Penang, Malaysia; [email protected] 4 Department of Emergency Medicine, Universiti Kebangsaan Malaysia Medical Centre, 56000 Kuala Lumpur, Malaysia; [email protected] 5 Thai Red Cross Society, Queen Saovabha Memorial Institute, Bangkok 10330, Thailand; [email protected] (S.K.); [email protected] (V.S.) * Correspondence: [email protected] Received: 31 December 2018; Accepted: 30 January 2019; Published: 6 February 2019 Abstract: Trimeresurus nebularis is a montane pit viper that causes bites and envenomation to various communities in the central highland region of Malaysia, in particular Cameron’s Highlands. To unravel the venom composition of this species, the venom proteins were digested by trypsin and subjected to nano-liquid chromatography-tandem mass spectrometry (LC-MS/MS) for proteomic profiling. Snake venom metalloproteinases (SVMP) dominated the venom proteome by 48.42% of total venom proteins, with a characteristic distribution of P-III: P-II classes in a ratio of 2:1, while P-I class was undetected. -

Venomous Snakes
Venomous Snakes - By Kedar Bhide Kedar Bhide is a snake expert from Mumbai. A postgraduate from Mumbai's Haffkine Institute, his work has resulted into first records of 2 snake species for India, Barta (Kaulback's Pit Viper) from Arunachal Pradesh and the Sind Awl-headed snake from Rajasthan. “ Moments after being bitten, the man feels a live fire germinating in the wound as if red hot tongs contorted his flesh; that which was mortified enlarges to monstrosity, and lividness invades him. The unfortunate victim witnesses his body becoming corpse piece by piece; a chill of death invades all his being, and soon bloody threads fall from his gums; and his eyes, without intending to, will also cry blood, until, beaten by suffering and anguish, he loses the sense of reality. If we then ask the unlucky man something, he may see us through blurred eyes, but we get no response; and perhaps a final sweat of red pearls or a mouthful of blackish blood warns of impending” (This is an introduction of a book written in 1931 by a Costa Rican Biologists and snakebite expert Clodomiro Picado.) INTRODUCTION Human fear of snakes is caused almost entirely by those species that can deliver a venomous bite. It is somewhat ironic that such a minority group, like venomous snakes has endangered the whole kingdom of snakes. Let us start by correcting a frequent misnomer. People often refer to poisonous snakes, and indeed by directory definition, this is not incorrect. But as a student of herpetology we should be more specific in our terminology. -

Skewed Sex Ratio of the Chinese Green Tree Viper, Trimeresurus Stej
Zoological Studies 42(2): 379-385 (2003) Skewed Sex Ratio of the Chinese Green Tree Viper, Trimeresurus stej- negeri stejnegeri, at Tsaochiao, Taiwan Shiuang Wang, Hua-Ching Lin and Ming-Chung Tu* Department of Biology, National Taiwan Normal University, Taipei, Taiwan 116, R.O.C. (Accepted December 5, 2002) Shiuang Wang, Hua-Ching Lin and Ming-Chung Tu (2003) Skewed sex ratio of the Chinese green tree viper, Trimeresurus stejnegeri stejnegeri, at Tsaochiao, Taiwan. Zoological Studies 42(2): 379-385. Preliminary collect- ing of adult Chinese green tree vipers, Trimeresurus stejnegeri stejnegeri, in Tsaochiao, northwestern Taiwan, yielded a male-biased sample. According to the sex ratio theory, a skewed sex ratio is unlikely at birth, and the above results may thus reflect sampling bias or differential mortality after birth. We collected litters of neonates of this viviparous snake from a broader area over a longer period to examine causes of such numerical domi- nance of males in the adult sample. We collected 23 gravid females from the Tsaochiao area in 3 consecutive reproductive seasons and obtained 50 male and 40 female neonates. The sex ratio at birth was not significant- ly biased. We marked and released a total of 169 male and 79 female T. s. stejnegeri. Marked snakes were recaptured 940 times. Regardless of season, time of day, transect, or sample area, we always encountered more males than females. Thus, sampling bias did not likely account for this skewed sex ratio. In the adult sample, the ratio of males was greatest in the largest size class, and this suggests that females are subjected to higher mortality. -

Red List of Bangladesh 2015
Red List of Bangladesh Volume 1: Summary Chief National Technical Expert Mohammad Ali Reza Khan Technical Coordinator Mohammad Shahad Mahabub Chowdhury IUCN, International Union for Conservation of Nature Bangladesh Country Office 2015 i The designation of geographical entitles in this book and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, International Union for Conservation of Nature concerning the legal status of any country, territory, administration, or concerning the delimitation of its frontiers or boundaries. The biodiversity database and views expressed in this publication are not necessarily reflect those of IUCN, Bangladesh Forest Department and The World Bank. This publication has been made possible because of the funding received from The World Bank through Bangladesh Forest Department to implement the subproject entitled ‘Updating Species Red List of Bangladesh’ under the ‘Strengthening Regional Cooperation for Wildlife Protection (SRCWP)’ Project. Published by: IUCN Bangladesh Country Office Copyright: © 2015 Bangladesh Forest Department and IUCN, International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holders, provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holders. Citation: Of this volume IUCN Bangladesh. 2015. Red List of Bangladesh Volume 1: Summary. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh, pp. xvi+122. ISBN: 978-984-34-0733-7 Publication Assistant: Sheikh Asaduzzaman Design and Printed by: Progressive Printers Pvt. -

Trimeresurus Venom Inhibition of Anti-HPA-1A and Anti-HPA-1B Antibody Binding to Human Platelets
Trimeresurus venom inhibition of anti-HPA-1a and anti-HPA-1b antibody binding to human platelets S.J. WLODAR, D.L. STONE, AND L.T. SINOR A solid-phase red cell adherence assay was used to demonstrate the platelet aggregation procedures, immobilization of specific inhibitory effect of seven species of Trimeresurus snake platelet monolayers in a solid-phase red cell adherence venom on the binding of HPA-1a- and HPA-1b-specific platelet anti- 4 bodies. Trimeresurus venom did not inhibit the binding of HLA-, (SPRCA) assay was used to measure the inhibitory HPA-3a-, HPA-3b-, HPA-4a-, HPA-5a-, and HPA-5b-specific platelet anti- effect of Trimeresurus venoms on glycoprotein IIb-IIIa- bodies. Venom from other genera of snakes, including representa- specific antibody binding. tives from Agkistrodon, Ancistrodon, Bitis, Bothrops, Bungarus, Causus, Crotalus, Dendroaspis, Ecis, Micrurus, Naja, Notechis, Ophiophagus, Pseudechis, Sepedon (Hemachatus), and Vipera, all Materials and Methods failed to specifically inhibit anti-HPA-1a and HPA-1b binding. These One hundred frozen human plasma and serum sam- results may indicate that the component in Trimeresurus snake ples, with previously determined platelet antibody venom previously reported to bind to the platelet GPIIb-IIIa complex, inhibiting fibrinogen binding, binds close to the HPA-1a and HPA-1b specificities, were thawed and retested by SPRCA to epitopes. Immunohematology 1995;11:129–132. confirm their reaction strengths and specificity (Capture-P Ready Screen, Immucor, Inc., Norcross, Snake venoms are composed of complex mixtures of GA). All test sample reactions were scored indepen- proteins that have many different biological effects on dently by two investigators, and only those samples mammalian cardiac and circulatory systems. -
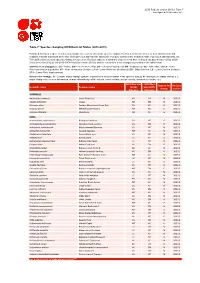
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

AMERICAN MUSEUM NOVITATES Published by the American Mueum Op NATURAL History May 1933 Number 620 New York City 13
AMERICAN MUSEUM NOVITATES Published by THE AmERICAN Mueum oP NATURAL HISToRY May 1933 Number 620 New York City 13, 59.81, 26 T (504) A STUDY OF THE GREEN PIT-VIPERS OF SOUTHEASTERN ASIA AND MALAYSIA, COMMONLY IDENTIFIED AS TRIMERESURUS GRAMINEUS (SHAW), WITH DESCRIPTION OF A NEW SPECIES FROM PENINSULAR INDIA' BY CLIFFORD H. POPE AND SARAH H. POPE This paper is based primarily upon the large number of pit-vipers in the collection of the British Museum (Natural History) and that of The American Museum of Natural History. Its object is to show that no less than five species are commonly identified as Trimeresurus gramineus and also to prove that these five species not only are distinct structurally but geographically or ecologically as well. It has long been known that certain green species of Trimeresurus are not readily separated from one another, and a study of the literature makes it evident that all but one of the forms treated here have been more or less understood by previous workers, but it is equally evident that their relationships have never been worked out or set forth by any one herpetologist. The species described as new below has been con- sistently confused with gramineus even though it is not even closely allied to that or any other species dealt with herein. A study of the hemipenis of nearly every valid species of Tri- meresurus has convinced us that this genus may be divided into groups of allied forns having different types of hemipenes. A simple key will suffice to show that the six species described in this paper possess no less than three distinct types of structure in this organ. -

Ecography ECOG-04374 Chen, C., Qu, Y., Zhou, X
Ecography ECOG-04374 Chen, C., Qu, Y., Zhou, X. and Wang, Y. 2019. Human overexploitation and extinction risk correlates of Chinese snakes. – Ecography doi: 10.1111/ecog.04374 Supplementary material 1 Human overexploitation and extinction risk correlates of Chinese snakes 2 3 Supporting information 4 Appendix 1. Properties of the datasets used, hypotheses and justification 5 Table A1. Extinction risk and predictor variables of Chinese snakes 6 Table A2. Hypotheses on the relationship between extinction risk and intrinsic and extrinsic factors 7 Table A3. Main sources for assessing elevational range and human exploitation index 8 Table A4. The correlation matrices of predictor variables for each snake group 9 Table a5. The full AICc models for Chinese snakes 10 Table a6. The interactions between geographic range size and other important variables 11 Appendix 2. Patterns of extinction risk using the IUCN Red List criteria 12 Appendix 3. Distribution of extinction risk among snake genera 13 Appendix 4. Correlates of extinction risk when species classified under range-based criteria were excluded 14 15 16 17 Appendix 1. Properties of the datasets used, hypotheses and justification 18 Table A1. Extinction risk (based on China Biodiversity Red List), intrinsic traits and extrinsic factors of Chinese snakes. Abbreviations: China, 19 China Biodiversity Red List; RangeC, species assessed under range-based criteria; IUCN, IUCN Red List; BL, Body length; BR, Body ratio; 20 AP, Activity period; MH, Microhabitat; RM, Reproductive mode; HS, Habitat -

ECOLOGY and CONSERVATION of PIT VIPERS ALONG the WESTERN GHATS (GOA) THESIS Submitted to GOA UNIVERSITY
ECOLOGY AND CONSERVATION OF PIT VIPERS ALONG THE WESTERN GHATS (GOA) THESIS Submitted to GOA UNIVERSITY FOR THE AWARD OF DEGREE OF DOCTOR OF PHILOSOPHY IN ZOOLOGY BY NITIN S. SAWANT DEPARTMENT OF ZOOLOGY GOA UNIVERSITY GOA 403 206 FEBRUARY 201 1 ECOLOGY AND CONSERVATION OF PIT VIPERS ALONG THE WESTERN GHATS, (GOA) THESIS SUBMITTED TO GOA UNIVERSITY FOR THE AWARD OF DEGREE OF DOCTOR OF PHILOSOPHY IN ZOOLOGY 557 , 563 BY c4) Eco NITIN S. SAWANT DEPARTMENT OF ZOOLOGY GOA UNIVERSITY GOA 403 206 FEBRUARY 2011 T- 508 DECLARATION [ hereby declare that the work reported in the present thesis entitled "Ecology and Conservation )f Pit Vipers along the Western Ghats (Goa)" is solely carried out by me under the supervision )f Dr. S. K. Shyama, Department of Zoology, Goa University, Taleigao, Plateau Goa. To the )est of my knowledge, the present study is the first comprehensive work of its kind from the area nentioned. No part thereof has been submitted for any other degree or diploma of this university )r any other University/Institute. 'lace: Goa University (Nitin S. Sawant) )ate: \ 9_0\1 Research student CERTIFICATE This is to certify that the thesis entitled "Ecology and Conservation of Pit Vipers along the Western Ghats (Goa)" submitted by Mr. Nitin S. Sawant for the award of the degree of Doctor of Philosophy in Zoology, is based on his original and independent work carried out by him during he period of study, under my supervision. Ile thesis or any part thereof has not been previously submitted for any other degree or diploma n any University or Institute.