۸۵.The Hydrochemical Evaluation of Groundwater Quality in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter Four Introduction to The
CHAPTER FOUR INTRODUCTION TO THE STUDY AREA 4.1 Introduction This chapter provides a general picture of socio-economic life in the four villages selected for the case study. All four villages are located in Northwestern Iran and, in terms of administrative divisions, belong to the Central District of the county of Marand. To give a general picture of the area where the sample villages are located, the present chapter begins with an overview of the socio-economic features of the county of Marand. This discussion is followed by a brief review of the villages' spatial context. Two types of data are used to describe and explain demographic changes in the sample villages. Changes in the size and structure of the population during the 2.5 years from 1966 till 1991 come from census data. Iran has experienced a massive and historically unprecedented movement of people from rural to urban centres over the past few decades (Kazemi 1980; Ayremlo 1984a; Hesamian, Etemad and Haery 1934; Hosseinzadeh 1992; Lahsaeizadeh, 1993b). In the context of the study area, this will be demonstrated by using data from the field survey. Infrastructure and provision of services will also be discussed to show the extent of the availability of needed services for residents on the one hand and the diversity among sample villages in terms of access on the other. This part of the chapter is based on field observations and data colleci:ed from interviews with local informed people. The final section of the Chapter concludes the discussion. 4.2 Study area in administrative divisions The four villages selected for the study of socio-economic changes are situated in the Dowlat Abad Subdistrict of the county of Marand in the province of Eastern 76 Azarbaijan (Map 4.1). -

New Species Ofastragalus L. (Leguminosae)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Annalen des Naturhistorischen Museums in Wien Jahr/Year: 2004 Band/Volume: 105B Autor(en)/Author(s): Podlech D. Artikel/Article: New species of Astragalus L. (Leguminosae), mainly from Iran. 565-596 ©Naturhistorisches Museum Wien, download unter www.biologiezentrum.at Ann. Naturhist. Mus. Wien 105 ß 565 - 596 Wien, April 2004 New species of Astragalus L. (Leguminosae), mainly from Iran D. Podlech* Abstract During the work on Astragalus for "Flora Iranica" and the neighbouring countries 34 new species of Astragalus could be discovered among the rich material collected in recent times. They belong to the fol- lowing sections: Sect. Brachylobium: A. abditus, A. aestivorum, A. bojnurdensis; Sect. Caprini: A. attarae; Sect. Dissitiflori: A. aestimabilis, A. submontanus; Sect. Erioceras: A. nubicola; Sect. Hymenostegis: A. austrokhorasanicus, A. vernaculus; Sect. Hypoglottidei: A. dengolanensis; Sect. Incanì: A. alavaanus, A. caespititius, A. divandarrehensis, A. diversus,A. dopolanicus,A. gulul-saranii, A. kuhidashtensis, A. nal- bandicus, A. paucifoliolatus; Sect. Malacothrix: A. angustistipulatus, A. bajgiranensis, A. brevitomentosus, A. heterophyllus, A. marandicus, A. mutuus, A. roessleri, A. sarikamishensis, A. shuturunkuhensis; Sect. Mesocarpon: A. pseudoquisqualis; Sect. Onobrychoidei: A. sufianicus; Sect. Plagiophaca: A. turgidus; Sect. Polycladus: A. pseudobagramiensis; Sect. Stereothrix: A. pseudocapito; Sect. Trachycercis: A. pseudo- shebarensis. A. pseudopendulina KAMELE is renamed because of illegitimacy: A. neplii. Keywords: Leguminosae, Astragalus; Flora of Iran; new species. Zusammenfassung Im Verlauf der Bearbeitung der Gattung Astragalus für die "Flora Iranica" und die angrenzenden Gebiete konnten 34 neue Arten der Gattung aufgefunden werden, die hier beschrieben werden. Sie gehören zu folgenden Sektionen: Sect. -

Saudi Arabia Iraq
40°0’0"E 42°30’0"E 45°0’0"E 47°30’0"E Yalnizbag Gasankend Sovetlyar Chakhar Gëkharli Te rc a n Anitli Balik Golu Suveren DZHERMUK Martuni Moranly Cayirli Hacibekir Eleskirt Eleskirtcilikân Karaköse Igdir Ararat Ararat Kyarki Vayots Dzor Gndevaz Pichanis Nagorno-karabakh Bejladjan Imamverdili Vtoroye Erzincan ERZINCAN Tepsicik Ahura Ger-Ger Saatly Sädäräk Malishka Armenia Zhdanovsk AkhmedliChakhirly Areni Ghai-kend Borisovka Minkend Armenia Bozoglak Alpkoy Pencirikkomu Te km a n Karayazi Tasliçay Golyuzu Golu Soylan Shusha Farsabad Frunze Tanyeri Günesgören Girberan AzerbaijanAzerbaijan Latchin Imichli Öztürkân Azatek Azizbekov Kargin Magara Haciomer Ilich Dyg Kemah Erzincan Caglayan Gokoglan Tutak Almalu Angekhakot Sisian AzerbaijanAzerbaijan Ordzhonikidze Bilesuvar Agri Diyadin Charchibogan Bartsruni Karacan Dogubayazit Zarkatun Kirovka Pulumur Erzurum Cherur Goris Aslanduz Novograzhdanovka Eskigedik Kasikli Karaseyidali Kagnili Khandek Fizuli Chalmeh Sürbahan Bazargan Pus’yan Karabaglar Chakhbuz Tazagyukh Syunik Goradiz Eleskirt Babash Kandi Pushkino Agalykend Ovacik Balpayam Aktuzla Pirzeynel Sust Dzhagry Kargapazar Qendeal Shakhtakhty Shakhbuz Dastakert Tatev Kubatly Bilesevar Hinis Binpinar Maku Kubatly Moradlu Tazakend Sowf’ali Arafsa Djebrail Larijan Balkiri Karaoglan Holik Bileci Baglarpinari Çatma Bebek ArmeniaArmenia Caylar Patnos Vodokhranilishche Gidrouzla Araks Khanlyk Soltanly Djalilabad Novogolovka Nazimiye Karliova Bayro Tujipazar Alikeykhaly Var to Malazgirt Bayazitaga Dash Feshel Nakhichevan’ Djulfa Shabadin Kafan Tunceli -

Water and Electricity
IRAN STATISTICAL YEARBOOK 1391 8. WATER AND ELECTRICITY Introduction Aquatic year: see Chapter 1, Definitions and he statistics appeared in this chapter have concepts. been provided as register records by Water produced: the amount of water gained from the Ministry of Energy on two topics of various (surface and underground) water resources "water" and "electricity". such as wells, springs, subterranean canals, dams T and river basins. 1. Water Dam: a structure built against the flow of water to This section includes information on reserve water or change the direction of flow or "underground waters", "reservoir dams", and manage it for satisfying different needs such as "length of networks and number of water and drinking, industry, irrigation (agriculture), sewage extensions". The related statistics have electricity generation and control of flood. been added to the Statistical Yearbook of Iran Reservoir dam: a dam made for reserving, since the year 1346. managing or controlling the flow of water to Statistics on underground waters and reservoir reserve it for procuring water for irrigation, dams have been provided by Water Resources drinking, industry, electricity generation and Management Company and statistics on the length control of flood of networks and number of water and sewage Large reservoir dam: refers to all dams with a extensions has been obtained from the Water and height of 15 metres or more as well as 10 to 15 Sewage Engineering Company. metres high dams having a reservoir with a Central and Internal basin, Hamun basin, and volume of 1 million cubic metres or more and/or Sarakhs basin were renamed by Water Resources a capacity of flood discharge of 2000 or more Management Organization as Central Plateau, cubic metres per second. -

Water and Electricity
IRAN STATISTICAL YEARBOOK 1394 9. WATER AND ELECTRICITY Introduction Aquatic year: see Chapter 1, Definitions and he statistics appeared in this chapter have concepts. Tbeen provided as register records by the Ministry Water produced: the amount of water gained from of Energy on two topics of "water" and various (surface and underground) water resources "electricity". such as wells, springs, subterranean canals, dams and river basins. Water Dam: a structure built against the flow of water to This section includes information on reserve water or change the direction of flow or "underground waters", "reservoir dams", and manage it for satisfying different needs such as "length of networks and number of water and drinking, industry, irrigation (agriculture), sewage extensions". The related statistics have electricity generation and control of flood. been added to the Statistical Yearbook of Iran Reservoir dam: a dam made for reserving, since the year 1346. managing or controlling the flow of water to Statistics on underground waters and reservoir reserve it for procuring water for irrigation, dams have been provided by Water Resources drinking, industry, electricity generation and Management Company and statistics on the length control of flood of networks and number of water and sewage Large reservoir dam: refers to all dams with a extensions has been obtained from the Water and height of 15 metres or more as well as 10 to 15 Sewage Engineering Company. metres high dams having a reservoir with a It is notworthy that Central and Internal basin, volume of 1 million cubic metres or more and/or a Hamun basin, and Sarakhs basin were renamed by capacity of flood discharge of 2000 or more cubic Water Resources Management Organization as metres per second. -

Medicago Sativa L.) Populations Using Morphological Traits and Rapd Markers
Journal of Applied Biological Sciences E-ISSN: 2146-0108 15(1): 91-100, 2021 Research Article DETECTING ALFALFA (MEDICAGO SATIVA L.) POPULATIONS USING MORPHOLOGICAL TRAITS AND RAPD MARKERS Hassan Monirifar 1*, Sajjad Moharramnejad 2 1 Crop and Horticultural Science Research Department, East Azerbaijan Agricultural and Natural Resources Research and Education Center, AREEO, Tabriz, Iran 2 Crop and Horticultural Science Research Department, Ardabil Agricultural and Natural Resources Research and Education Center, AREEO, Moghan, Iran *Corresponding Author: E-mail: [email protected] (Received 29th August 2020; accepted 23rd December2020) ABSTRACT. In order to evaluate several alfalfa populations from East Azerbaijan of Iran, an experiment based on randomized complete block design was performed under field conditions during three appropriate growing seasons (2010-2013) at the East Azarbaijan Agricultural and Natural Resources Research and Education Center, Tabriz, Iran. Thirty alfalfa populations were selected from East Azerbaijan, Iran. The morphological traits and molecular marker (RAPD) were evaluated in all alfalfa populations. The result showed that the most morphological attributes had a significant difference between alfalfa populations, and it was indicated that each alfalfa population is related to various locations of East Azerbaijan. Correlation drawn between dry weight and agronomic attributes in the alfalfa populations showed that dry weight was strongly correlated with plant height. Cluster analysis, using UPGMA procedure, based on RAPD banding pattern in 30 alfalfa populations formed four groups. Overall, all alfalfa populations had more genetic diversity and can be used in a breeding program that can be made synthetic cultivars. Key words: Alfalfa, Cluster, Diversity, Environment, Germplasm, Morphological. INTRODUCTION Alfalfa (Medicago sativa L.), a fundamental forage crop grown in the temperate regions, is cultivated over 32 million hectares worldwide [15] and about 680 thousand hectares in the northwest of Iran. -
Iran (Islamic Republic Of) Armenia a Zerbaijan
45°0’0"E 47°30’0"E 50°0’0"E Fiagdon Sunzhenskoye Nizhneye Klyuchevoye Lipovka Alkhazurovo Chiri-Yurt Benoy Tashi Bezhta Bol’shaya Shagada Gilyany Mugutala Akay-Bulak Shamkhal Alagir Gizel’ Komgaran Lugovoye Goichu Araderikh Betli Bil’ty Dylym Guni Gomulat-Tala Khataldon Dattykh Meredzhoi-Beram Grushevoye Agashty Makhach-Aul Ishtiburi Gendergen Chechel’-Yukh Khubar Zubutl’ Ekibulak Suadag Mayramadag KambileyevskoyeMuzhichi Duba-Yurt Kolob Biragzang Trëkhgor’ye AgashbatoyMokok Benoi Dakhada Baytarki Almak Burtunay Gerli Kizil-Yurt Makhachkala Dzuarikau Dzhaudzhikau Zavodskoy Oktyabr’skiy Cherkai Nizhniy Tamisk Detskoye Dachnoye Gurchi Ersenoy Gurki-Mokhk Alkhan Kapchugay Angusht Munzhgyan Gilech Razdol’noye Khvarshini Kirov-Yurt Tando Lyusu Atly-Boyun Tagardon Khabal Chernorechinskiy Chem-Aul Kartsa Redant Akabas Terkhi Vedeno Banoy-Vedeno Agchu Sikly-Kol Gusara Verkhniy Gerit Amki Dal’neye Maloye Bukuzi Dutsu-Khote Evli Makhi Agachaul Kilichnyy Koban Balta Bardabos Kyzyk Azivgus Bugut-Yurt Khay Gazuni Peshkhob Avarskoye Nui Akhatly Chichelau Kaspiysk Donisar Tergispira Sovetskoye Kharachoy Artlukh Kaptan Fortaukh Gerchoch Kerety Zandak-Irzu Leshkoroy Al’ma-Terek Tuar Verkhniy Fiagdon Dargavs Keli Barakh Nikota Keymekhki Dzhainakhoy Tumsoy Danukh Nizhniye Ishkarty Buynaksk Pardzhan Karmadon Beyna Khuli Khali-Keloy Gonti Pari-Aul Tsilit Kafyr-Kumuch Kakadur Dariali Chikaroy Arguani Verkhniy Karanay Dzheyrakh Tumga Besht Gul Burti Aslambek-Sheripovo Gagatl’ Nizhniy Karanay Atlan-Aul Yanashi Kharisdzhin Dzhimara Koka Kay Oshni Gukhoy Achaloy -

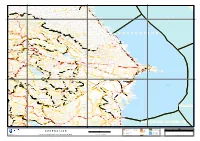
Investigation of Hydrochemical Characteristics of Groundwater in the Harzandat Aquifer, Northwest of Iran
Environ Monit Assess DOI 10.1007/s10661-010-1575-4 Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran Nosrat Aghazadeh · A. A. Mogaddam Received: 30 November 2009 / Accepted: 15 June 2010 © Springer Science+Business Media B.V. 2010 2+) − 2−) Abstract The Harzandat plain is part of the East Mg and weak acids (HCO3 ,CO3 .The Azerbaijan province, which lies between Marand chemical quality of groundwater is related to and Jolfa cities, northwestern of Iran, and its the dissolution of minerals, ion exchange, and groundwater resources are developed for water the residence time of the groundwater in contact supply and irrigation purposes. The main litho- with rock materials. The results of calculation sat- logic units consist chiefly of limestone, dolomite, uration index by computer program PHREEQC shale, conglomerate, marl, and igneous rocks. In shows that nearly all of the water samples were order to evaluate the quality of groundwater in supersaturated with respect to carbonate minerals study area, 36 samples were collected and an- (calcite, dolomite and aragonite) and undersatu- alyzed for various ions. Chemical indexes like rated with respect to sulfate minerals (gypsum and sodium adsorption ratio, percentage of sodium, anhydrite). Assessment of water samples from residual sodium carbonate, and permeability in- various methods indicated that groundwater in dex were calculated. Based on the analytical re- study area is chemically unsuitable for drinking sults, groundwater in the area is generally very and agricultural uses. hard, brackish, high to very high saline and al- kaline in nature. The abundance of the ma- Keywords Groundwater quality · − − 2− · · jor ions is as follows: Cl >HCO3 >SO4 and Harzandat plain Hydrochemical Na+>Ca2+>Mg2+>K+. -

Occurrence of Phoebodus Gothicus (Chondrichthyes: Elasmobranchii) in the Middle Famennian of Northwestern Iran (Province East Azerbaij An)
Acta Geologica Polonica, Vol. 50 (2000), No.3, pp. 355-367 Occurrence of Phoebodus gothicus (Chondrichthyes: Elasmobranchii) in the middle Famennian of northwestern Iran (Province East Azerbaij an) OLIVER HAMPE Museum [iir Naturkunde, Zentralinstitut der Humboldt-Universitiit zu Berlin, Institut [iir Paliiontologie, InvalidenstrafJe 43, D-I01l5 Berlin, Germany. E-mail: [email protected] ABSTRACT: HAMPE, O. 2000. Occurrence of Phoebodus gothicus (Chondrichthyes: Elasmobranchii) in the middle Famennian of northwestern Iran (Province East Azerbaijan). Acta Geologica Polonica, 50 (3), 355-367. Warszawa. Teeth of the stratigraphically relevant elasmobranch Phoebodus gothicus were recovered from samples collected from the Ilan-Ghareh Formation in the neighbourhood of Zonuz in East Azerbaijan province, Iran. The associated fauna confirms a middle Famennian age, marginilera and trachytera conodont Zone. Comparisons with European, North American, African and other Asian locations show a stratigraphically restricted distribution of Phoebodus gothicus in the Famennian, Early marginilera to Middle praesulcata Zone. The Zonuz sample furthermore contains conodonts, ostracods, gastropods, crinoids as well as tooth and bone fragments of other chondrichthyans, placoderms, and oste ichthyans. The ostracods indicate a neritic, shallow water environment with a probable high nutrient flux. Keywords: Elasmobranch teeth, Ilan-Ghareh Fm, Azarbayjan-e Sharqi, Associated fauna, Biostratigraphy and Distribution. INTRODUCTION The material presented in this paper was recov ered from a small sample collected in 1967 by a The elasmobranch genus Phoebodus is a common German-Iranian expedition of the Institute of form in the Middle and Late Devonian and Early Geosciences of the University of Mainz, Germany, Carboniferous which unfortunately, to date, is only known which went to different isles in the Aegean Sea over by its teeth. -

369 Introduction
IRAN STATISTICAL YEARBOOK 1390 8. WATER AND ELECTRICITY Introduction drinking, industry, irrigation (agriculture), electricity he statistics appeared in this chapter have generation and control of flood. Reservoir dam: a dam made for reserving, managing been provided as register records by the or controlling the flow of water to reserve it for Ministry of Energy on two topicsof "water" procuring water for irrigation, drinking, industry, and "electricity". T electricity generation and control of flood 1. Water Large reservoir dam: refers to all dams with a Thissection includes information on "underground height of 15 metres or more as well as 10 to 15 waters", "reservoir dams", and "length of networks and metres high dams having a reservoir with a number of water and sewage extensions". The related volume of 1 million cubic metres or more and/or a statistics have been added to the Statistical Yearbook capacity of flood discharge of 2000 or more cubic of Iran since the year 1346. Statistics on underground waters and reservoir dams metres per second. Inflow: annual volume of water entered the reservoir have been provided by Water Resources Management of a dam through the river. Company and statistics on the length of networks and Outflow: total annual volume of water discharged number of water and sewage extensions has been from different outlets of a dam (weir, silt ejector obtained from the Water and Sewage Engineering channels, take-out gates, drainage channels) and Company. evaporation. Central and Internal basin, Hamun basin, and Sarakhs Water extension: refers to the part of branched-off basin were renamed by Water Resources Management water pipes, containing pipe, related accessories, with Organization as Central Plateau, Eastern Border and a profile appropriate to the water metre and the Qareh Qum respectively, in the year 1383. -

Entomofauna Ansfelden/Austria; Download Unter
© Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Entomofauna ZEITSCHRIFT FÜR ENTOMOLOGIE Band 30, Heft 10: 137-152 ISSN 0250-4413 Ansfelden, 2. Jänner 2009 A brief study on the tribes Tortricini and Archipini (Lepidoptera: Tortricinae) from Iran Helen ALIPANAH Abstract Thirty-three species of the tribes Tortricini and Archipini are listed. Previous studies dealing with the tribes are summarized and new data on their distribution in Iran are provided. The genera Aleimma and Argyrotaenia and the species Aleimma loeflingiana (LINNAEUS), Acleris rhombana (DENIS & SCHIFFERMÜLLER), A. quercinana (ZELLER), A. sparsana (DENIS & SCHIFFERMÜLLER), A. lacordairana caucasica FILIPJEV, A. forsskaleana (LINNAEUS), Pandemis heparana (DENIS & SCHIFFERMÜLLER), Argyrotaenia ljungiana (THUNBERG), Choristoneura lafauryana (RAGONOT), Archips crataeganus (HÜBNER), A. philippa (MEYRICK), Aphelia ochreana HÜBNER, A. viburnana (DENIS & SCHIFFERMÜLLER) and A. peramplana (HÜBNER) are newly reported from Iran. Introduction The subfamily Tortricinae is a paraphyletic group, but there are no synapomorphies to associate particular tribes within the subfamily. This subfamily consists of 11 tribes, many of which are distributed in the Palaearctic region with some (Sparganothini, Eulini) having only a few representatives. So far, more than 2600 species belonging to 372 genera have been described in the Tortricinae, of which most belong to two tribes, the Cochylini and Archipini (HORAK 1999). 137 © Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at -

S. A. Rafat, Phd
University of Tabriz [email protected] Department of Animal Science S. A. Rafat, PhD Research Experience Feb 2016 – present Professor (Full) University of Tabriz, Department of Animal Science Tabrīz, Iran Jun 2011 – Feb 2016 Associate Professor University of Tabriz, Department of Animal Science Tabrīz, Iran Jun 2007 – Jun 2011 Professor (Assistant) University of Tabriz, Department of Animal Science Tabrīz, Iran Jan 2004 – Jun 2007 Doctorant ( PhD Student) French National Institute for Agricultural Research (INRA), INPT - Institut National Polytechnique de Toulouse (France) Toulouse, Midi-Pyrénées, France La Station d'Amélioration Génétique des Animaux (SAGA, UR631) a été créée en 1970 suite à la décentralisation du département de Génétique Animale (GA) et à la création du Centre de Toulouse. Jun 1998 – Jan 2004 Lecturer University of Tabriz, Tabrīz, East Azarbaijan, Iran Awards & Grants Feb 2011 Grant: Coordinated Research Project on “Genetic Variation on the Control of Resistance to Infectious Diseases in Small Ruminants for Improving Animal Productivity” Skills & Activities Skills Animal Science, Animal Breeding, Genetics, Selection, Quantitative Genetics, Inbreeding, Asreml, Animals, Animal Genomics, Mastitis, Dairy Cattle, Fiber, SAS Base, SAS/STAT, divergent selection, Milk, Animal Genetics, Cattle Breeding, Animal Production, Animal Biotechnology, Dairy Science, Coding Languages English, French, Turkish Publication Highlights Hajializadeh R, Abbas Rafat S, Notter DR, Djalil Shojda, Gholamali Moghaddam, Ahmad Nematollahi: Fecal egg counts for gastrointestinal nematodes are associated with a polymorphism in the MHC-DRB1 gene in the Iranian Ghezel sheep breed. Frontiers in Genetics 02/2015; 6., DOI:10.3389/fgene.2015.00105 S A Rafat, P Namavar, D J Shodja, H Janmohammadi, H Z Khosroshahi, I David: Estimates of the genetic parameters of Turkey body weight using Random Regression analysis.