Validation Method for Assessing Intensity of Mistletoe Infestation in Teak
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disturbances Influence Trait Evolution in Pinus
Master's Thesis Diversify or specialize: Disturbances influence trait evolution in Pinus Supervision by: Prof. Dr. Elena Conti & Dr. Niklaus E. Zimmermann University of Zurich, Institute of Systematic Botany & Swiss Federal Research Institute WSL Birmensdorf Landscape Dynamics Bianca Saladin October 2013 Front page: Forest of Pinus taeda, northern Florida, 1/2013 Table of content 1 STRONG PHYLOGENETIC SIGNAL IN PINE TRAITS 5 1.1 ABSTRACT 5 1.2 INTRODUCTION 5 1.3 MATERIAL AND METHODS 8 1.3.1 PHYLOGENETIC INFERENCE 8 1.3.2 TRAIT DATA 9 1.3.3 PHYLOGENETIC SIGNAL 9 1.4 RESULTS 11 1.4.1 PHYLOGENETIC INFERENCE 11 1.4.2 PHYLOGENETIC SIGNAL 12 1.5 DISCUSSION 14 1.5.1 PHYLOGENETIC INFERENCE 14 1.5.2 PHYLOGENETIC SIGNAL 16 1.6 CONCLUSION 17 1.7 ACKNOWLEDGEMENTS 17 1.8 REFERENCES 19 2 THE ROLE OF FIRE IN TRIGGERING DIVERSIFICATION RATES IN PINE SPECIES 21 2.1 ABSTRACT 21 2.2 INTRODUCTION 21 2.3 MATERIAL AND METHODS 24 2.3.1 PHYLOGENETIC INFERENCE 24 2.3.2 DIVERSIFICATION RATE 24 2.4 RESULTS 25 2.4.1 PHYLOGENETIC INFERENCE 25 2.4.2 DIVERSIFICATION RATE 25 2.5 DISCUSSION 29 2.5.1 DIVERSIFICATION RATE IN RESPONSE TO FIRE ADAPTATIONS 29 2.5.2 DIVERSIFICATION RATE IN RESPONSE TO DISTURBANCE, STRESS AND PLEIOTROPIC COSTS 30 2.5.3 CRITICAL EVALUATION OF THE ANALYSIS PATHWAY 33 2.5.4 PHYLOGENETIC INFERENCE 34 2.6 CONCLUSIONS AND OUTLOOK 34 2.7 ACKNOWLEDGEMENTS 35 2.8 REFERENCES 36 3 SUPPLEMENTARY MATERIAL 39 3.1 S1 - ACCESSION NUMBERS OF GENE SEQUENCES 40 3.2 S2 - TRAIT DATABASE 44 3.3 S3 - SPECIES DISTRIBUTION MAPS 58 3.4 S4 - DISTRIBUTION OF TRAITS OVER PHYLOGENY 81 3.5 S5 - PHYLOGENETIC SIGNAL OF 19 BIOCLIM VARIABLES 84 3.6 S6 – COMPLETE LIST OF REFERENCES 85 2 Introduction to the Master's thesis The aim of my master's thesis was to assess trait and niche evolution in pines within a phylogenetic comparative framework. -

Abundance, Distribution, Population Structure, and Substrate Use of Ambystoma Altamirani Along the Arroyo Los Axolotes, State of Mexico, Mexico
Herpetological Conservation and Biology 15(1):188–197. Submitted: 16 August 2019; Accepted: 23 February 2020; Published: 30 April 2020. ABUNDANCE, DISTRIBUTION, POPULATION STRUCTURE, AND SUBSTRATE USE OF AMBYSTOMA ALTAMIRANI ALONG THE ARROYO LOS AXOLOTES, STATE OF MEXICO, MEXICO VIRIDIANA VILLARREAL HERNÁNDEZ1, GEOFFREY R. SMITH2, RAYMUNDO MONTOYA AYALA3, AND JULIO A. LEMOS-ESPINAL1,4 1Laboratorio de Ecología - Unidad de Biotecnología y Prototipos, Facultad de Estudios Superiores Iztacala, Avendina Los Barrios 1, Los Reyes Iztacala, Tlalnepantla, Estado de México, 54090, México 2Department of Biology, Denison University, Granville, Ohio 43023, USA 3Laboratorio de Cómputo - Unidad de Biotecnología y Prototipos, Facultad de Estudios Superiores Iztacala, Avenida Los Barrios 1, Los Reyes Iztacala, Tlalnepantla, Estado de México, 54090, México 4Corresponding author: e-mail: [email protected] Abstract.—Ambystomatid salamanders in central Mexico are confronted by anthropogenic threats that can limit their distribution and abundance. Ambystoma altamirani (Mountain Stream Siredon) is listed as Endangered by the International Union for Conservation of Nature (IUCN) Red List and as Threatened by the Mexican government. We report on the distribution, abundance, occupancy, population structure, and substrate use of A. altamirani, a stream dwelling salamander, along the Arroyo los Axolotes, Sierra de las Cruces, Mexico. We observed A. altamirani at least once during repeated surveys between February 2018 to December 2018 in 24 of 25 permanent 5-m long reaches separated by 40 m. The best model for occupancy had constant occupancy, detection, extinction, and colonization probabilities. Sites that dried at some time during the study had fewer observed individuals than those that did not dry. Size structure was relatively constant throughout the year, except for the appearance of small larvae in May, June, and July. -

Estado Del Arte De Las Plantas Parásitas En México
MMS ESTADO DEL ARTE DE PLANTAS PARÁSITAS EN MÉXICO CONSEJO NACIONAL DE CIENCIA Y TECNOLOGÍA RED TEMÁTICA DE SALUD FORESTAL COMISIÓN NACIONAL FORESTAL UNIVERSIDAD AUTÓNOMA CHAPINGO COLEGIO DE POSTGRADUADOS ESTADO DEL ARTE DE LAS PLANTAS PARÁSITAS EN MÉXICO Líder de la Línea de Investigación: Dr. Dionicio Alvarado Rosales. Postgrado en Fitosanidad-Fitopatología Colegio de Postgraduados Campus Montecillo, Edo. de México. DICIEMBRE, 2016 Ilustraciones: Mauricio Méndez S. Portada: Psittacanthus angustifolius Contenido: fruto de Cladocolea cupulata (Tomadas de: Geils et al., 2002). Página 2 ESTADO DEL ARTE DE PLANTAS PARÁSITAS EN MÉXICO CONTENIDO Pág. INTRODUCCIÓN 4 ANTECEDENTES 5 RELATORÍA DEL FORO 6 1. 1. IDENTIFICACIÓN DE ESPECIES 13 2. 2. ESPECIFICIDAD 18 3. 3. BIOLOGÍA MOLECULAR 18 4. 4. IMPACTO (PATOGÉNICO, ECONÓMICO Y SOCIAL) 19 5. 5. ESTUDIOS HISTOPATOLÓGICOS 23 6. 6. DIAGNÓSTICO E INCIDENCIA 24 7. 7. CONTRIBUCIÓN A LOS CICLOS BIOLÓGICOS 26 8. 8. ESTUDIOS EPIDEMIOLÓGICOS 28 9. 9. ESTRATEGIAS DE CONTROL Y MANEJO 29 10. NORMATIVIDAD 35 LITERATURA CITADA 41 ANEXOS 50 Página 3 MMS ESTADO DEL ARTE DE PLANTAS PARÁSITAS EN MÉXICO INTRODUCCIÓN Por cientos de años, las plantas parásitas han ocupado el interés público (religioso y mítico), y desde el siglo pasado los científicos investigan y estudian sus efectos en especies forestales de importancia económica en diversas partes del mundo. A pesar de que poseen pigmentos fotosensibles, estas plantas tienen hábitos parasitarios, por lo que dependen parcial o completamente de su hospedante para satisfacer sus demandas nutrimentales. Sus raíces modificadas (haustorios) les brindan soporte (fijación), y el vínculo que les permite extraer de su hospedante los suficientes recursos (carbohidratos, agua y sales minerales) para completar sus complejos ciclos biológicos. -

Anatomía De La Madera De Tres Especies De Mimosa
doi:10.21829/myb.2017.2311518 Madera y Bosques vol. 23, núm. 1: 39-51 Primavera 2017 Composición y diversidad de especies forestales en bosques templados de Puebla, México Composition and diversity of forest species in forests temperate of Puebla, Mexico Juan Antonio López-Hernández1, Óscar A. Aguirre-Calderón1, Eduardo Alanís-Rodríguez1*, José Carlos Monarrez-Gonzalez2, Marco A. González-Tagle1 y Javier Jiménez-Pérez1 1 Universidad Autónoma de Nuevo León. Facultad de 2 Centro Interdisciplinario de Investigación para el De- * Autor de correspondencia. eduardo.alanisrd@uanl. Ciencias Forestales. Linares, Nuevo León, México. sarrollo Integral Regional, Unidad Durango. Durango, edu.mx Dgo., México. RESUMEN La presente investigación se realizó con el objetivo de evaluar la composición y la diversidad de las especies forestales en bosques tem- plados en la zona este del estado de Puebla (centro de México). La base de datos se obtuvo de 45 sitios permanentes de investigación silvícola (SPIS), ubicados en la región forestal Centro y Pico de Orizaba pertenecientes a la Unidad de Manejo Forestal, Umafor 2105. Se registró información dasométrica de altura total (m), diámetro a la altura del pecho (cm) y cobertura de copa de ejemplares con un diámetro a la altura del pecho mayor o igual a 7.5 cm. Para cada una de las especies se cuantificó su dominancia a través del área basal, su abundancia de acuerdo con el número de árboles y su frecuencia en las parcelas de muestreo. Se generó un valor ponderado para cada especie, denominado índice valor de importancia (IVI). La diversidad y la riqueza de especies se estimaron utilizando el índice de diversidad de Shannon-Wiener (H´) y el índice de Margalef (DMg). -
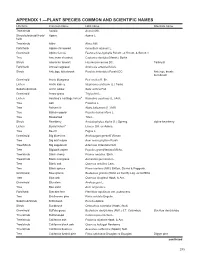
APPENDIX 1.—PLANT SPECIES COMMON and SCIENTIFIC NAMES Life Form Common Name Latin Name Alternate Name Tree/Shrub Acacia Acacia Mill
APPENDIX 1.—PLANT SPECIES COMMON AND SCIENTIFIC NAMES Life form Common name Latin name Alternate name Tree/shrub Acacia Acacia Mill. Shrub/subshrub//Forb/ Agave Agave L. herb Tree/shrub Alder Alnus Mill. Forb/herb Alpine chickweed Cerastium alpinum L. Graminoid Alpine fescue Festuca brachyphylla Schult. ex Schult. & Schult. f. Tree American chestnut Castanea dentata (Marsh.) Borkh. Shrub American tarwort Flourensia cernua DC. Tarbrush Forb/herb Annual ragweed Ambrosia artemisiifolia L. Shrub Antelope bitterbrush Purshia tridentata (Pursh) DC. Antelope brush; buckbrush Graminoid Arctic bluegrass Poa arctica R. Br. Lichen Arctic kidney Nephroma arcticum (L.) Torss. Subshrub/shrub Arctic willow Salix arctica Pall. Graminoid Arrow grass Triglochin L. Lichen Asahina’s cartilage lichend Ramalina asahinae (L.) Ach. Tree Ash Fraxinus L. Tree Balsam fi r Abies balsamea (L.) Mill. Tree Balsam poplar Populus balsamifera L. Tree Basswood Tilia L. Shrub Bearberry Arctostaphylos alpina (L.) Spreng. alpine bearberry Lichen Beard lichena Usnea Dill. ex Adans. Tree Beech Fagus L. Graminoid Big bluestem Andropogon gerardii Vitman Tree Big leaf maple Acer macrophyllum Pursh Tree/Shrub Big sagebrush Artemisia tridentata Nutt. Tree Bigtooth aspen Populus grandidentata Michx. Tree/shrub Black cherry Prunus serotina, Ehrh. Tree/shrub Black mangrove Avicennia germinans L. Tree Black oak Quercus velutina, Lam. Tree Black spruce Picea mariana (Mill.) Britton, Sterns & Poggenb. Graminoid Blue grama Bouteloua gracilis (Willd. ex Kunth) Lag. ex Griffi ths Tree Blue oak Quercus douglasii Hook. & Arn. Graminoid Bluestem Andropogon L. Tree Box elder Acer negundo L. Forb/herb Bracken fern Pteridium aquilinum var. pubescens Tree Bristlecone pine Pinus aristata Engelm. Subshrub/Shrub Brittlebush Encelia Adans. -

El Niosouthern Oscillation Effect on a Fire Regime in Northeastern Mexico Has Changed Over Time
Ecology, 91(6), 2010, pp. 1660–1671 Ó 2010 by the Ecological Society of America El Nin˜o–Southern Oscillation effect on a fire regime in northeastern Mexico has changed over time 1,6 1 2 3 3 LARISSA L. YOCOM, PETER Z. FULE´, PETER M. BROWN, JULIA´N CERANO, JOSE´VILLANUEVA-DI´AZ, 4 5 DONALD A. FALK, AND ELADIO CORNEJO-OVIEDO 1School of Forestry and Ecological Restoration Institute, Northern Arizona University, P.O. Box 15018, Flagstaff, Arizona 86011-5018 USA 2Rocky Mountain Tree-Ring Research, Incorporated, 2901 Moore Lane, Ft. Collins, Colorado 80526 USA 3Instituto Nacional de Investigaciones Forestales, Agrı´colas, y Pecuarias, Centro Nacional de Investigacio´n Disciplinaria en Relacio´n Agua, Suelo, Planta, Atmo´sfera, Gomez Palacio, Durango 35140 Mexico 4School of Natural Resources and Laboratory of Tree-Ring Research, Biological Sciences East, University of Arizona, Tucson, Arizona 85721 USA 5Departamento Forestal, Universidad Auto´noma Agraria ‘‘Antonio Narro,’’ Saltillo, Coahuila, Mexico Abstract. The El Nin˜o Southern Oscillation (ENSO) is a climate-forcing mechanism that has been shown to affect precipitation and the occurrence of wildfires in many parts of the world. In the southern United States and northern Mexico, warm events (El Nin˜o) are associated with moist winter conditions and fewer fires, while cool events (La Nin˜a) tend to favor dry winters and more fires. We tested this relationship in a region of northeastern Mexico by characterizing the historical fire regime and climatic influences. Fire regimes were reconstructed from fire-scar samples collected from 100 trees in three high-elevation sites on Pen˜a Nevada in southern Nuevo Leo´n. -

5 Estudio Florístico En El Área De La Comunidad
Acta Botanica Mexicana (2000), 52: 5-41 ESTUDIO FLORÍSTICO EN EL ÁREA DE LA COMUNIDAD INDÍGENA DE NUEVO SAN JUAN PARANGARICUTIRO, MICHOACÁN, MÉXICO1,2 CONSUELO MEDINA GARCÍA, FERNANDO GUEVARA-FÉFER MARCO ANTONIO MARTÍNEZ RODRÍGUEZ, PATRICIA SILVA-SÁENZ, MA. ALMA CHÁVEZ-CARBAJAL Facultad de Biología Universidad Michoacana de San Nicolás de Hidalgo 58060 Morelia, Michoacán E IGNACIO GARCÍA RUIZ3 CIIDIR – IPN Michoacán Justo Sierra 28 59510 Jiquilpan, Michoacán RESUMEN El estudio florístico realizado en el área de la comunidad indígena de Nuevo San Juan Parangaricutiro registró la presencia de 108 familias, con 307 géneros, 716 especies y 16 taxa infraespecíficos, de los cuales, 52 son helechos y afines, 16 gimnospermas, 120 monocotiledóneas y 544 dicotiledóneas. Las familias mejor representadas son: Compositae (135), Leguminosae (58), Gramineae (57), Labiatae (26), Solanaceae (21) Orchidaceae (20) y Polypodiaceae (18). 60.7% de las especies corresponden a la forma de vida herbácea (perenne y anual), 19.1% son arbustos, 10.0% árboles, 4.2% trepadoras, 3.3% epífitas, 1.8% parásitas, “saprófitas” 0.5% y acuáticas 0.4%. ABSTRACT The inventory of the vascular flora in the area of comunidad indígena de Nuevo San Juan Parangaricutiro produced the following results: 108 families with 307 genera, 716 species and 16 infraespecific taxa. From this total 52 species belong to pteridophytes, 16 to gymnosperms, 120 to monocotyledons and 544 to dicotyledons. The best represented families, in terms of species number are: Compositae (135), Leguminosae (58), Gramineae (57), Labiatae (26), Solanaceae (21), Orchidaceae (20), and Polypodiaceae (18). 60.7% of the species are herbaceous (either perennial or annual plants); 19.1% are shrubs, 10.0% trees, 4.2% lianas, 3.3% are epiphytic plants, 1.8% are parasites, “saprophytes” amount to 0.5% and aquatics 0.4%. -

Mistletoes of North American Conifers
United States Department of Agriculture Mistletoes of North Forest Service Rocky Mountain Research Station American Conifers General Technical Report RMRS-GTR-98 September 2002 Canadian Forest Service Department of Natural Resources Canada Sanidad Forestal SEMARNAT Mexico Abstract _________________________________________________________ Geils, Brian W.; Cibrián Tovar, Jose; Moody, Benjamin, tech. coords. 2002. Mistletoes of North American Conifers. Gen. Tech. Rep. RMRS–GTR–98. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 123 p. Mistletoes of the families Loranthaceae and Viscaceae are the most important vascular plant parasites of conifers in Canada, the United States, and Mexico. Species of the genera Psittacanthus, Phoradendron, and Arceuthobium cause the greatest economic and ecological impacts. These shrubby, aerial parasites produce either showy or cryptic flowers; they are dispersed by birds or explosive fruits. Mistletoes are obligate parasites, dependent on their host for water, nutrients, and some or most of their carbohydrates. Pathogenic effects on the host include deformation of the infected stem, growth loss, increased susceptibility to other disease agents or insects, and reduced longevity. The presence of mistletoe plants, and the brooms and tree mortality caused by them, have significant ecological and economic effects in heavily infested forest stands and recreation areas. These effects may be either beneficial or detrimental depending on management objectives. Assessment concepts and procedures are available. Biological, chemical, and cultural control methods exist and are being developed to better manage mistletoe populations for resource protection and production. Keywords: leafy mistletoe, true mistletoe, dwarf mistletoe, forest pathology, life history, silviculture, forest management Technical Coordinators_______________________________ Brian W. Geils is a Research Plant Pathologist with the Rocky Mountain Research Station in Flagstaff, AZ. -

The Evolution of Cavitation Resistance in Conifers Maximilian Larter
The evolution of cavitation resistance in conifers Maximilian Larter To cite this version: Maximilian Larter. The evolution of cavitation resistance in conifers. Bioclimatology. Univer- sit´ede Bordeaux, 2016. English. <NNT : 2016BORD0103>. <tel-01375936> HAL Id: tel-01375936 https://tel.archives-ouvertes.fr/tel-01375936 Submitted on 3 Oct 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE BORDEAUX Spécialité : Ecologie évolutive, fonctionnelle et des communautés Ecole doctorale: Sciences et Environnements Evolution de la résistance à la cavitation chez les conifères The evolution of cavitation resistance in conifers Maximilian LARTER Directeur : Sylvain DELZON (DR INRA) Co-Directeur : Jean-Christophe DOMEC (Professeur, BSA) Soutenue le 22/07/2016 Devant le jury composé de : Rapporteurs : Mme Amy ZANNE, Prof., George Washington University Mr Jordi MARTINEZ VILALTA, Prof., Universitat Autonoma de Barcelona Examinateurs : Mme Lisa WINGATE, CR INRA, UMR ISPA, Bordeaux Mr Jérôme CHAVE, DR CNRS, UMR EDB, Toulouse i ii Abstract Title: The evolution of cavitation resistance in conifers Abstract Forests worldwide are at increased risk of widespread mortality due to intense drought under current and future climate change. -

Photo Series for Quantifying Forest Fuels in Mexico Is a Tool for Quickly 1984)6
University of Fotoseries para la Cuantificación de Combustibles Washington Forestales de México: College of Forest Resources Bosques Montanos Subtropicales de la Sierra Madre del Sur y Bosques Templados y Matorral Submontano del Norte de la Sierra Madre Oriental Month 2007 Photo Series for Quantifying Forest Fuels in México: Montane Subtropical Forests of the Sierra Madre del Sur, and Temperate Forests and Montane Shrubland of the Northern Sierra Madre Oriental United States Forest Service Pacific Northwest Jorge E. Morfín-Ríos, Ernesto Alvarado-Celestino, Enrique J. Jardel-Peláez, Research Station Robert E. Vihnanek, David K. Wright, José M. Michel-Fuentes, Clinton S. Wright, Roger D. Ottmar, David V. Sandberg & Andrés Nájera-Díaz. Universidad de Guadalajara United States Agency for International Development Fondo Mexicano para la Conservación de la Naturaleza Fotoseries para la Cuantificación de Combustibles Forestales de University of México: Washington Bosques Montanos Subtropicales de la Sierra Madre del Sur y College of Forest Resources Bosques Templados y Matorral Submontano del Norte de la Sierra Madre Oriental Photo Series for Quantifying Forest Fuels in México: Month 2007 Montane Subtropical Forests of the Sierra Madre del Sur, and Temperate Forests and Montane Shrubland of the Northern Sierra Madre Oriental Jorge E. Morfín-Ríos, Ernesto Alvarado-Celestino, Enrique J. Jardel-Peláez, Robert E. Vihnanek, David K. Wright, José M. Michel-Fuentes, Clinton S. Wright, Roger D. Ottmar, David V. Sandberg & Andrés Nájera-Díaz. United States Forest Service Pacific Northwest Research Station Universidad de Guadalajara United States Agency for International Development Fondo Mexicano para la Conservación de la Naturaleza RESUMEN ABSTRACT Morfín Ríos, J.E.; Alvarado Celestino, E.; Jardel Peláez, E.J.; Vihnanek, Morfín Ríos, J.E.; Alvarado Celestino, E.; Jardel Peláez, E.J.; Vihnanek, R.E.; Wright, D.K.; Michel Fuentes, J.M.; Wright, C.S.; Ottmar, R.D.; R.E.; Wright, D.K.; Michel Fuentes, J.M.; Wright, C.S.; Ottmar, R.D.; Sandberg, D.V.; Nájera Díaz, A. -

Growth of Hartweg's Pine (Pinus Hartwegii) Parasitized
Botanical Sciences 94 (1): 51-62, 2016 ECOLOGY DOI: 10.17129/botsci.218 GROWTH OF HARTWEG’S PINE (PINUS HARTWEGII) PARASITIZED BY TWO DWARF MISTLETOE SPECIES (ARCEUTHOBIUM SPP.) MÓNICA E. QUEIJEIRO-BOLAÑOS AND ZENÓN CANO-SANTANA1 Departamento de Ecología y Recursos Naturales, Facultad de Ciencias, Universidad Nacional Autónoma de México, México D.F., Mexico 1Corresponding author: [email protected] Abstract: Coniferous forests occupy a large extent (17 %) of the Mexican territory. Within these forests, pines are a common and sometimes dominant component; however, several abiotic and biotic factors affect pines growth. Among the main biotic factors is the parasitic effect of dwarf mistletoes. In Zoquiapan (Iztaccíhuatl Popocatépetl National Park, Central Mexico) two dwarf mistletoe species coexist parasitizing Pinus hartwegii. The aim of this study was to know the effect of Arceuthobium globosum and A. vaginatum, either individually or as a pair, on P. hartwegii growth, allometric relations, and size susceptibility. We recorded diameter at breast height (dbh) and crown spread of P. hartwegii for 3 years on individuals infested by either one of the species, both, or none, as well as the infection severity. The relative growth rate (RGR) in diameter was strongly infuenced by the pines initial dbh; whereas the infecting species or severity did not show a differential effect. The allometric relation of dbh and height was affected by parasitism, where the trees infected by both species were shorter than the uninfected and infected by A. vaginatum at the same dbh. The parasitic effect does not differ among these mistletoe species.,However, the host-size structure affects the presence and severity of infection; maintaining even-age stands provides a better scenario for a milder effect of parasitism, which should be considered for managing plans. -

Diversity and Origin of the Central Mexican Alpine Flora
diversity Article Diversity and Origin of the Central Mexican Alpine Flora Victor W. Steinmann 1, Libertad Arredondo-Amezcua 2, Rodrigo Alejandro Hernández-Cárdenas 3 and Yocupitzia Ramírez-Amezcua 2,* 1 Facultad de Ciencias Naturales, Universidad Autónoma de Querétaro, Av. de las Ciencias s/n, Del. Sta. Rosa Jáuregui, Querétaro 76230, Mexico; [email protected] or [email protected] 2 Private Practice, Pátzcuaro, Michoacán 61600, Mexico; [email protected] 3 Herbario Metropolitano, División de Ciencias Biológicas y de la Salud, Departamento de Biología, Universidad Autónoma Metropolitana-Iztapalapa, Avenida San Rafael Atlixco #186, Colonia Vicentina, Iztapalapa, Ciudad de México 09340, Mexico; [email protected] * Correspondence: [email protected] Abstract: Alpine vegetation is scarce in central Mexico (≈150 km2) and occurs on the 11 highest peaks of the Trans-Mexican Volcanic Belt (TMVB). Timberline occurs at (3700) 3900 m, and at 4750 m vascular plants cease to exist. The alpine vascular flora comprises 237 species from 46 families and 130 genera. Asteraceae (44), Poaceae (42), and Caryophyllaceae (21) possess 45% of the species; none of the remaining families have more than 10 species. Four species are strict endemics, and eight others are near endemics. Thirteen species are restricted to alpine vegetation but also occur outside the study area. Seventy-seven species are endemic to Mexico, 35 of which are endemic to the TMVB. In terms of biogeography, the strongest affinities are with Central or South America. Fifteen species are also native to the Old World. Size of the alpine area seems to not be the determining factor for its floristic diversity. Instead, the time since and extent of the last volcanic activity, in addition to the distance from other alpine islands, appear to be important factors affecting diversity.