Anetoderma: a Case Report and Review of the Literature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UC Davis Dermatology Online Journal
UC Davis Dermatology Online Journal Title Penicillamine-associated cutis laxa and milia en plaque - case report and review of cutaneous changes associated with penicillamine Permalink https://escholarship.org/uc/item/47p4d8zv Journal Dermatology Online Journal, 22(5) Authors Vajdi, Tina Lee, Wiggin Wu Paravar, Taraneh Publication Date 2016 DOI 10.5070/D3225030951 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Volume 22 Number 5 May 2016 Photo Vignette Penicillamine-associated cutis laxa and milia en plaque - case report and review of cutaneous changes associated with penicillamine Tina Vajdi1, Wiggin Wu Lee2, Taraneh Paravar2 Dermatology Online Journal 22 (5): 12 1University of California, San Diego School of Medicine 2Department of Dermatology, University of California, San Diego Correspondence: Taraneh Paravar, MD Assistant Clinical Professor Department of Dermatology University of California, San Diego 8899 University Center Lane, Suite 350 San Diego, California 92122, USA Tel. (858) 657-8322 E-mail: [email protected] Abstract Penicillamine-induced skin changes are rare and include: hypersensitivity reactions, autoimmune reactions, and cutaneous elastoses. We report a case of a 73-year-old man with cystinuria taking penicillamine for over 50 years who presented with penicillamine-induced cutis laxa and milia en plaque. A brief review of penicillamine induced skin changes, specifically cutis laxa and milia en plaque, is presented. Key Words: penicillamine, elastic tissue, cystinuria, cutis laxa, milia en plaque Introduction Penicillamine is a chelating agent commonly used to treat cystinuria and Wilson disease. Cystinuria is a genetic disorder in which patients lack the cysteine amino acid transporter. -
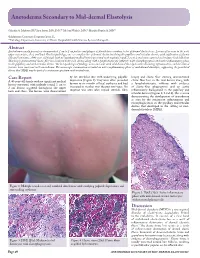
Anetoderma Secondary to Mid-Dermal Elastolysis
Anetoderma Secondary to Mid-dermal Elastolysis Gabriela A. Maloney, BS,* Jane James, MD, PhD,** Michael Welsch, MD,** Marylee Braniecki, MD** *Midwestern University, Downers Grove, IL **Pathology Department, University of Illinois Hospital & Health Sciences System, Chicago, IL Abstract Anetoderma usually presents as circumscribed, 1 cm to 2 cm patches and plaques of flaccid skin secondary to loss of dermal elastic tissue. Lesions often occur in the neck, upper extremities, chest, and back. On histopathology, one sees complete loss of dermal elastin involving the papillary and reticular dermis, with infiltration of plasma cells and histiocytes. A 40-year-old female with no significant medical history presented with multiple round, 1 cm to 2 cm lesions scattered on her upper back and chest. Skin biopsy demonstrated elastic-fiber loss localized to the mid-dermis along with a lymphohistiocytic infiltrate with elastophagocytosis and active inflammatory phase in the papillary and mid-reticular dermis. The histopathological findings were consistent with mid-dermal elastolysis with advancing inflammation, and the clinical features were consistent with anetoderma. The microscopic examination revealed an active inflammatory phase of mid-dermal elastolysis, supporting the postulated theory that MDE may be part of a continuous spectrum with anetoderma. Case Report by lax, wrinkled skin with underlying palpable biopsy and elastic-fiber staining demonstrated A 40-year-old female with no significant medical depression (Figure 1). They were often preceded elastic-fiber loss in the mid-dermis along with history presented with multiple round, 1 cm to by two to six months of local erythema and had a lymphohistiocytic infiltrate with evidence 2 cm lesions scattered throughout the upper increased in number over the past two years. -

Dermatose Degenerativa Induzida Por D-Penicilamina Em Paciente Com Doença De Wilson
Revista SPDV 76(2) 2018; D-Penicillamine induced degenerative dermopathy; Rui Pedro Santos, Joana Gomes, Celeste Brito. Caso Clínico Dermatose Degenerativa Induzida por D-penicilamina em Paciente com Doença de Wilson Rui Pedro Santos1, Joana Gomes2, Celeste Brito2 1Interno de Dermatovenereologia/Resident, Dermatovenereology, Hospital de Braga, Braga, Portugal 2Especialista de Dermatovenereologia/Specialist of Dermatovenereology, Hospital de Braga, Braga, Portugal RESUMO – As dermatoses degenerativas induzidas por D-penicilamina incluem, entre outras, a elastose perfurante serpiginosa e o pseudo-pseudoxantoma elástico. A elastose perfurante serpiginosa é uma doença perfurante rara caracterizada pela elimi- nação transepidérmica de fibras elásticas anormais. Esta condição pode ser idiopática, reativa ou induzida por D-penicilamina, habitualmente utilizada para o tratamento da doença de Wilson, cistinúria, artrite reumatóide ou esclerose sistémica. Manifesta- ções cutâneas semelhantes a pseudoxantoma elástico mas sem história familiar e mutações do gene ABCC6 foram identificadas como sendo uma dermatose induzida por D-penicilamina e designada de pseudo-pseudoxantoma elástico. Descreve-se o caso de uma mulher de 17 anos tratada por vários anos com D-penicilamina para doença de Wilson, com pápulas assintomáticas, algumas cor de pele e hiperqueratósicas e outras macias e amareladas, na região cervical e face. A histopatolo- gia mostrou a eliminação transepidérmica de fibras elásticas espessadas, em forma de dentes de serra. Estes achados sugeriram uma dermopatia induzida por D-penicilamina e os autores consideraram o diagnóstico de elastose perfurante serpiginosa e pseudo-pseudoxantoma elástico no mesmo paciente. O fármaco foi alterado para acetato de zinco sem lesões novas, mas com manutenção das lesões existentes no seguimento a 1 ano. -

5 Allergic Diseases (And Differential Diagnoses)
Chapter 5 5 Allergic Diseases (and Differential Diagnoses) 5.1 Diseases with Possible IgE Involve- tions (combination of type I and type IVb reac- ment (“Immediate-Type Allergies”) tions). Atopic eczema will be discussed in a separate section (see Sect. 5.5.3). There are many allergic diseases manifesting in The maximal manifestation of IgE-mediated different organs and on the basis of different immediate-type allergic reaction is anaphylax- pathomechanisms (see Sect. 1.3). The most is. In the development of clinical symptoms, common allergies develop via IgE antibodies different organs may be involved and symp- and manifest within minutes to hours after al- toms of well-known allergic diseases of skin lergen contact (“immediate-type reactions”). and mucous membranes [also called “shock Not infrequently, there are biphasic (dual) re- fragments” (Karl Hansen)] may occur accord- action patterns when after a strong immediate ing to the severity (see Sect. 5.1.4). reactioninthecourseof6–12harenewedhy- persensitivity reaction (late-phase reaction, LPR) occurs which is triggered by IgE, but am- 5.1.1 Allergic Rhinitis plified by recruitment of additional cells and 5.1.1.1 Introduction mediators.TheseLPRshavetobedistin- guished from classic delayed-type hypersensi- Apart from being an aesthetic organ, the nose tivity (DTH) reactions (type IV reactions) (see has several very interesting functions (Ta- Sect. 5.5). ble 5.1). It is true that people can live without What may be confusing for the inexperi- breathing through the nose, but disturbance of enced physician is familiar to the allergist: The this function can lead to disease. Here we are same symptoms of immediate-type reactions interested mostly in defense functions against are observed without immune phenomena particles and irritants (physical or chemical) (skin tests or IgE antibodies) being detectable. -

Elastosis Perforans Serpiginosa: a D-Penicillamine Induced Dermatoses in a Patient with Wilson’S Disease
Article / Clinical Case Report Elastosis Perforans Serpiginosa: a D-penicillamine induced dermatoses in a patient with Wilson’s disease Swagatika Samala , Mukund Sablea How to cite: Samal S, Sable M. Elastosis Perforans Serpiginosa: a D-penicillamine induced dermatoses in a patient with Wilson’s disease. Autops Case Rep [Internet]. 2020 Apr-Jun;10(2):e2020167. https://doi.org/10.4322/acr.2020.167 ABSTRACT Long term use of D-penicillamine for Wilson’s disease can be associated with many adverse reactions and systemic side effects. We report the case of a 28-year-old male patient diagnosed with Wilson’s disease presenting with a serpiginous raised violaceous skin lesion in the anterior aspect of the neck over the last six months and two small papules with central umbilication during the last month. Histopathological examination of skin lesions demonstrated transepidermal perforating channel, and the Verhoeff’s-van Gieson stain showed marked increase number of irregular serrated elastic fibers suggesting the diagnosis of D- penicillamine induced elastosis perforans serpiginosa. Keywords Skin Diseases; Biopsy; Elastic tissue. INTRODUCTION CASE REPORT D-penicillamine (DPA) therapy is the mainstay A 28-year-male diagnosed with WD on oral DPA of chelation therapy for patients of Wilson’s therapy (250 mg thrice daily) for the last 18 years disease (WD). Various systemic adverse effects, presented with serpiginous raised violaceous skin including many dermatological manifestations, lesions in the anterior aspect of neck over the last may be observed with prolonged use of this drug. six months and two small papules with central The dermatological side effects of DPA can be of three umbilication for one month (Figure 1). -

Striae Distensae
COMPARATIVE STUDY BETWEEN INTENSE PULSED LIGHT "IPL" AND PULSED DYE LASER IN THE TREATMENT OF STRIAE DISTENSAE Thesis Submitted for the Fulfillment of (Ph.D) Degree in Medical Applications of Laser By By Ghada Mohamed Kamal El-Din Ahmed El-Khalafawy (M.B.B.Ch., M.Sc.) & Diploma in Medical Laser Applications Under the supervision of Prof. Dr. Hisham Ali Shokeir Professor of Dermatology National Institute of Laser Enhanced Sciences – Cairo University Ass. Prof. Dr. Ahmed Fathy El-Bedewi Associate Professor of Dermatology Atomic Energy Authority Ass. Prof. Dr. Safinaz Salah El-Din Sayed Associate Professor of Histology Faculty of Medicine – Cairo University National Institute of Laser Enhanced Sciences Cairo University 2013 Approval Sheet COMPARATIVE STUDY BETWEEN INTENSE PULSED LIGHT "IPL" AND PULSED DYE LASER IN THE TREATMENT OF STRIAE DISTENSAE Thesis Submitted for the Fulfillment of (Ph.D) Degree in Medical Applications of Laser By By Ghada Mohamed Kamal El-Din Ahmed El-Khalafawy (M.B.B.Ch., M.Sc.) & Diploma in Medical Laser Applications Under the supervision of Prof. Dr. Hisham Ali Shokeir Ass. Prof. Dr. Ahmed Fathy El-Bedewi Ass. Prof. Dr. Safinaz Salah El-Din Sayed National Institute of Laser Enhanced Sciences Cairo University 2013 ﷲ ا ا ُ ْ ََ ُاا ُُ ْْ ََ َ َ ََ ََ ِِ ْ ََََ ََ ﱠﱠ ﱠﱠ ﱠﱠ ََ ْْ ِ ِ إإ ََ َ َ ْْ َ َ َ َ ََِِإإ ََأأ ََ ِِ ُُاا ْْ ََ ِِ ُاا ُ ق ﷲ ا رة اة ا ( 32) Acknowledgment I am deeply thankful to GODGOD, by the grace of whom, the present work was possible. -

Reticulate Dermatoses
[Downloaded free from http://www.e-ijd.org on Tuesday, April 08, 2014, IP: 111.93.251.154] || Click here to download free Android application for this journal CME Article Reticulate Dermatoses Keshavmurthy A Adya, Arun C Inamadar, Aparna Palit From the Department of Dermatology, Venereology and Leprosy, SBMP Medical College, Hospital and Research Center, BLDE University, Bijapur, Karnataka, India Abstract The term “reticulate” is used for clinical description of skin lesions that are configured in a net-like pattern. Many primary and secondary dermatoses present in such patterns involving specific body sites. Certain cutaneous manifestations of systemic diseases or genodermatoses also present in such manner. This review classifies and describes such conditions with reticulate lesions and briefly, their associated features. Key Words: Mottling, net-like, reticulate, retiform What was known? 3. Poikilodermatous Reticulate configuration of lesions is seen in many primary dermatoses and a. Inherited also as cutaneous reaction patterns consequent to internal pathology. • Rothmund–Thomson syndrome • Dyskeratosis congenita Reticulate Dermatoses • Xeroderma pigmentosum • Cockayne syndrome The term “reticulate” is commonly used for clinical • Fanconi anemia description of “net-like”, “sieve-like,” or “chicken wire” • Mendes da Costa syndrome configuration of the skin lesions. Various congenital • Kindler syndrome and acquired dermatoses present with this pattern of • Degos–Touraine syndrome skin lesions. Many systemic diseases also present with • Hereditary sclerosing poikiloderma of Weary such cutaneous manifestations providing useful clues to • Hereditary acrokeratotic poikiloderma of Weary diagnosis. • Werner’s syndrome (adult progeria) Classification • Chanarin–Dorfman syndrome • Diffuse and macular atrophic dermatosis 1. Vascular b. Acquired a. Cutis marmorata • Poikiloderma of Civatte b. -

Atrophic Erythematous Facial Plaques
Photo Challenge Atrophic Erythematous Facial Plaques What’s the diagnosis? A 26-year-old woman presented with a 2-year his- tory of facial lesions that had gradually increased in size and number. Initially they were tender and pruritic but eventually became asymptomatic. She denied aggravation with sun exposure and did not use regular sun protection. Multiple pulsed dye laser treatments to the lesions had not resulted in appreciable improvement. Review of systems revealed occasional blurred vision and joint pain in her wrist and fingers of her right hand. Physical examination revealed a healthy- CUTISappearing woman. On the forehead and bilateral cheeks there were multiple atrophic, erythematous, sunken plaques with discrete borders. Each plaque measured more than 5 mm. Similar plaques were scattered across the frontal scalp, trunk, and upper extremities, though fewer in number and less atrophic with mild hyperpigmentation. There was diffuse hair thinning of the scalp. Laboratory test results included a normal complete metabolic panel, anti- nuclear antibody profile, and complete blood cell count. DoHistopathology revealed Not a superficial and mid perivascular Copy and perifollicular inflammatory infiltrate composed of lymphocytes, histiocytes, and melanophages. Vacuolar changes in the dermoepidermal junction were present. There were few dyskeratotic keratinocytes and mucin deposition present in the dermis. Direct immunofluorescence was not performed. William S. Kaufman, MD; Elizabeth K. McNamara, MD; Rita Pichardo-Geisinger, MD From Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina. The authors report no conflict of interest. Correspondence: William S. Kaufman, MD, Wake Forest University Baptist Medical Center, Department of Dermatology, Medical Center Boulevard, Winston-Salem, NC 27157 ([email protected]). -

A Mechanochemical Model of Striae Distensae ⇑ Stephen J
Mathematical Biosciences 240 (2012) 141–147 Contents lists available at SciVerse ScienceDirect Mathematical Biosciences journal homepage: www.elsevier.com/locate/mbs A mechanochemical model of striae distensae ⇑ Stephen J. Gilmore a, Benjamin L. Vaughan Jr. b, , Anotida Madzvamuse c, Philip K. Maini b,d a Dermatology Research Centre, University of Queensland, School of Medicine, Princess Alexandra Hospital, Brisbane, Australia b Centre for Mathematical Biology, Mathematical Institute, University of Oxford, UK c Department of Mathematics, University of Sussex, UK d Oxford Centre for Integrative Systems Biology, Department of Biochemistry, University of Oxford, UK article info abstract Article history: Striae distensae, otherwise known as stretch marks, are common skin lesions found in a variety of clinical Received 6 October 2011 settings. They occur frequently during adolescence or pregnancy where there is rapid tissue expansion Received in revised form 28 June 2012 and in clinical situations associated with corticosteroid excess. Heralding their onset is the appearance Accepted 29 June 2012 of parallel inflammatory streaks aligned perpendicular to the direction of skin tension. Despite a consid- Available online 14 July 2012 erable amount of investigative research, the pathogenesis of striae remains obscure. The interpretation of histologic samples – the major investigative tool – demonstrates an association between dermal lympho- Keywords: cytic inflammation, elastolysis, and a scarring response. Yet the primary causal factor in their aetiology is Stretch marks mechanical; either skin stretching due to underlying tissue expansion or, less frequently, a compromised Mathematical modelling Numerical simulation dermis affected by normal loads. In this paper, we investigate the pathogenesis of striae by addressing the coupling between mechanical forces and dermal pathology. -

Atypical Acrodermatitis Chronica Atrophicans Herxheimer
www.symbiosisonline.org Symbiosis www.symbiosisonlinepublishing.com Case Report Clinical Research in Dermatology: Open Access Open Access Atypical Acrodermatitis Chronica Atrophicans Herxheimer Wollina U1*, Boldt S1, Heinig B2, Schönlebe J3 1Department of Dermatology and Allergology 2Center of Physical and Rehabilitative Medicine 3Institute of Pathology “Georg Schmorl”, Academic Teaching Hospital Dresden-Friedrichstadt, Dresden, Germany Received: December 14, 2015; Accepted: December 19, 2015; Published: December 23, 2015 *Corresponding author: Prof. Dr. U. Wollina, Department of Dermatology and Allergology, Academic Teaching Hospital Dresden-Friedrichstadt, Friedrichstrasse 41, 01067 Dresden, Germany. E-mail: [email protected] Abstract Acrodermatitis Chronica Atrophicans Herxheimer (ACA) is Sensitivity and specificity of enzyme immuno assay and immune a tick-born disease due to infection by Borrelia afzelii, the major blot are 95% and 80-95% for ACA [4]. Polymerase chain reaction vector organism is Ixodes rhicinus. We report on a 48-year-old male (PCR) of skin biopsies was positive in up to 88% on fresh-frozen tissueCase butReport only in 44-52% using paraffin-embedded tissue [5]. symmetric plaques associated with hyperpigmented widely distributedpatient who lesions developed within extensive the tension livid-erythematous lines, and acrocyanosis. fibrosclerotic The of a skin biopsy and laboratory investigations with positive IgG and A 48-year-old male patient was referred to our hospital IgMdiagnosis immunoblots. of ACA has The been patient confirmed was treated by histopathologic by intravenous examination ceftriaxone because of large livid-erythematous fibrosclerotic plaques on resulting in partial remission of cutaneous and extracutaneous his trunk and extremities which developed within half a year. He symptoms. suffered from arterial hypertension and had a penicillin allergy. -

Resident's Page
Resident’s Page SScarscars iinn ddermatology:ermatology: CClinicallinical signisignifi ccanceance BB.. AAnitha,nitha, SS.. RRagunatha,agunatha, AArunrun CC.. IInamadarnamadar Department of Dermatology, Venereology and Leprosy, BLDEA’s SBMP Medical College, Hospital and Research Centre, Bijapur, Karnataka, India AAddressddress fforor ccorrespondenceorrespondence : Dr. Arun C. Inamadar, Professorand Head, Department of Dermatology, Venereology and Leprosy, BLDEA’s SBMP Medical College, Hospital and Research Centre, Bijapur - 586103, Karnataka, India. E-mail:[email protected] [2] A scar is a scar is a scar and only a scar if you don’t ask ß1 protects the collagen from degradation. why” - Shelly and Shelly CCLASSIFICATIONLASSIFICATION OOFF SSCARSCARS[[3]3] A scar is a fibrous tissue replacement that develops as a 1. Fine line scars: Surgical scars consequence of healing at the site of a prior ulcer or 2. Wide (stretched) scars: These develop when fine wound. Cutaneous scarring is a macroscopic disturbance of line surgical scars gradually become stretched the normal structure and function of the skin architecture and widened. They are typically flat, pale, soft, manifesting itself as an elevated or depressed area, with an symptomless scars. Abdominal striae of pregnancy alteration of skin texture, color, vascularity, nerve supply can be considered as variants of these. [1] and biomechanical properties. 3. Atrophic scars: These are flat or depressed below the surrounding skin. They are generally small and Histologically, dermal scars are characterized by thickened often round with an indented or inverted centre. epidermis with a flattened dermo-epidermal junction and They commonly arise after acne or chickenpox. an abnormal organization of the dermal matrix into parallel 4. -

Global Development Assistance for Adolescent Health from 2003 to 2015
Supplementary Online Content Li Z, Li M, Patton GC, Lu C. Global development assistance for adolescent health from 2003 to 2015. JAMA Netw Open. 2018;1(4):e181072. doi:10.1001/jamanetworkopen.2018.1072 eTable 1. List of Donor Countries Included in the CRS eTable 2. 132 Recipients in the CRS (According to the World Health Organization Regions) eTable 3. Key Words to Identify the Related Age Group (Adolescence) in the Creditor Reporting System eTable 4. CRS Purpose Name and Respective Fractions Allocated to Adolescent Health eTable 5. Definitions of DAAH on the Leading Causes of DALYs of Adolescent Health eTable 6. Key Words Used to Search for Projects on Skin and Subcutaneous Diseases in the Creditor Reporting System eTable 7. Key Words Used to Search for Road Injury Projects in the Creditor Reporting System eTable 8. Key Words Used to Search for HIV/AIDS Projects in the Creditor Reporting System eTable 9. Key Words Used to Search for Projects on Iron-Deficiency Anemia in the Creditor Reporting System eTable 10. Key Words Used to Search for Self-Harm Projects in the Creditor Reporting System eTable 11. Key Words Used to Search for Projects on Interpersonal Violence in the Creditor Reporting System eTable 12. Key Words Used to Search for Projects on Depressive Disorders in the Creditor Reporting System eTable 13. Key Words Used to Search for Projects on Lower Back and Neck Pain in the Creditor Reporting System eTable 14. Key Words Used to Search for Diarrheal Projects in the Creditor Reporting System eTable 15. Key Words Used to Search for Tuberculosis Projects in the Creditor Reporting System eTable 16.