Ontogeny and Allometry of Body Shape in the Blacktail Shiner, Cyprinella Venusta
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Survey for Calhoun, Gordon County, Georgia
Blacktail Redhorse (Moxostoma poecilurum) from Oothkalooga Creek Fish Survey for Calhoun, Gordon County, Georgia Prepared by: DECATUR, GA 30030 www.foxenvironmental.net January 2018 Abstract Biological assessments, in conjunction with habitat surveys, provide a time-integrated evaluation of water quality conditions. Biological and habitat assessments for fish were conducted on 3 stream segments in and around Calhoun, Gordon County, Georgia on October 3 and 5, 2017. Fish, physical habitat, and water chemistry data were evaluated according to Georgia Department of Natural Resources (GADNR), Wildlife Resources Division (WRD) – Fisheries Section protocol entitled “Standard Operating Procedures for Conducting Biomonitoring on Fish Communities in Wadeable Streams in Georgia”. All of the water quality parameters at all sites were within the typical ranges for streams although conductivity was somewhat high across the sites. Fish habitat scores ranged from 80 (Tributary to Oothkalooga Creek) to 132.7 (Oothkalooga Creek). Native fish species richness ranged from 6 species (Tributary to Oothkalooga Creek) to 17 (Oothkalooga and Lynn Creeks). Index of biotic integrity (IBI) scores ranged from 16 (Tributary to Oothkalooga Creek; “Very Poor”) to 34 (Lynn Creek; “Fair”). Overall, the results demonstrate that Oothkalooga and Lynn Creeks are in fair condition whereas the Tributary to Oothkalooga Creek is highly impaired. Although the data are only a snapshot of stream conditions during the sampling events, they provide a biological characterization from which to evaluate the effect of future changes in water quality and watershed management in Calhoun. We recommend continued monitoring of stream sites throughout the area to ensure that the future ecological health of Calhoun’s water resources is maintained. -

Pennington Creek Fish
FAMILY: CENTRARCHIDAE (sunfishes) FAMILY: CYPRINIDAE (minnows) Bluegill Orangespotted Sunfish Smallmouth Bass Bigeye Shiner Lepomis macrochirus Lepomis humilis Micropterus dolomieu Notropis boops Characteristics: deep-bodied, small mouth, Characteristics: small with orange spots Characteristics: large mouth, vertical dark Characteristics: large eye relative to black spot posterior dorsal rays on side, long white-edged opercular flap bars are sometimes present on olive- body size, large mouth with a small bronze colored sides of the fish, juveniles head, dark lateral stripe extends from have an orange and black band on the cau- the lips through the eye to the end of dal fin the caudal peduncle Green Sunfish Redear Sunfish Largemouth Bass Blacktail Shiner Lepomis cyanellus Lepomis microlophus Micropterus salmoides Cyprinella venusta Characteristics: elongated body, large Characteristics: large, short opercular flap Characteristics: large mouth, upper jaw Characteristics: prominent black spot at mouth, black spot posterior dorsal & anal with a bright red crescent marking extends past the eye, dark midlateral the base of the caudal fin, large stripe from snout to base of the caudal fin diamond shaped scales outlined in black, breeding males develop yellow fins Longear Sunfish White & Black Crappie Spotted Bass Bluntnose Minnow Lepomis megalotis Pomoxis annularis, Pomoxis nigromacula- Micropterus punctulatus Pimephales notatus Characteristics: small, deep-bodied, long tus Characteristics: resembles the largemouth Characteristics: blunt, rounded -

Life History Plasticity of the Blacktail Shiner (Cyprinella
LIFE HISTORY PLASTICITY OF THE BLACKTAIL SHINER (CYPRINELLA VENUSTA) ACROSS DISTURBANCE GRADIENTS IN ALABAMA STREAMS Except where reference is made to the work of others, the work described in this thesis is my own or was done in collaboration with my advisory committee. This thesis does not include proprietary or classified information. ______________________________ Lemuel Robles Casten Certificate of approval: __________________________ __________________________ George W. Folkerts Carol E. Johnston, Chair Professor Associate Professor Biological Sciences Fisheries and Allied Aquacultures __________________________ __________________________ Russell A. Wright Stephen L. McFarland Associate Professor Acting Dean Fisheries and Allied Aquacultures Graduate School LIFE HISTORY PLASTICITY OF THE BLACKTAIL SHINER (CYPRINELLA VENUSTA) ACROSS DISTURBANCE GRADIENTS IN ALABAMA STREAMS Lemuel Robles Casten A Thesis Submitted to the Graduate Faculty of Auburn University in Partial Fulfillment of the Requirements for the Degree of Master of Science Auburn, Alabama August 7, 2006 LIFE HISTORY PLASTICITY OF THE BLACKTAIL SHINER (CYPRINELLA VENUSTA) ACROSS DISTURBANCE GRADIENTS IN ALABAMA STREAMS Lemuel Robles Casten Permission is granted to Auburn University to make copies of this thesis at its direction, upon request of individuals or institutions and at their expense. The author reserves all publication rights. __________________________ Signature of Author __________________________ Date of Graduation iii VITA Lemuel Robles Casten, was born in Iloilo City, Philippines on February 21, 1975. He attended the University of the Philippines in the Visayas and graduated with a Bachelor of Science degree in Fisheries in 1997. After graduation, he worked as fisheries officer for the International Marinelife Alliance and was research assistant for FishBase and LarvalBase Projects of the WorldFish Center from 1999-2004. -
![Kyfishid[1].Pdf](https://docslib.b-cdn.net/cover/2624/kyfishid-1-pdf-1462624.webp)
Kyfishid[1].Pdf
Kentucky Fishes Kentucky Department of Fish and Wildlife Resources Kentucky Fish & Wildlife’s Mission To conserve, protect and enhance Kentucky’s fish and wildlife resources and provide outstanding opportunities for hunting, fishing, trapping, boating, shooting sports, wildlife viewing, and related activities. Federal Aid Project funded by your purchase of fishing equipment and motor boat fuels Kentucky Department of Fish & Wildlife Resources #1 Sportsman’s Lane, Frankfort, KY 40601 1-800-858-1549 • fw.ky.gov Kentucky Fish & Wildlife’s Mission Kentucky Fishes by Matthew R. Thomas Fisheries Program Coordinator 2011 (Third edition, 2021) Kentucky Department of Fish & Wildlife Resources Division of Fisheries Cover paintings by Rick Hill • Publication design by Adrienne Yancy Preface entucky is home to a total of 245 native fish species with an additional 24 that have been introduced either intentionally (i.e., for sport) or accidentally. Within Kthe United States, Kentucky’s native freshwater fish diversity is exceeded only by Alabama and Tennessee. This high diversity of native fishes corresponds to an abun- dance of water bodies and wide variety of aquatic habitats across the state – from swift upland streams to large sluggish rivers, oxbow lakes, and wetlands. Approximately 25 species are most frequently caught by anglers either for sport or food. Many of these species occur in streams and rivers statewide, while several are routinely stocked in public and private water bodies across the state, especially ponds and reservoirs. The largest proportion of Kentucky’s fish fauna (80%) includes darters, minnows, suckers, madtoms, smaller sunfishes, and other groups (e.g., lam- preys) that are rarely seen by most people. -

BLUE SHINER Scientific Name: Cyprinella Caerulea Other
Common Name: BLUE SHINER Scientific Name: Cyprinella caerulea Other Commonly Used Names: none Previously Used Scientific Names: none Family: Cyprinidae Rarity Ranks: G2/S1 State Legal Status: Endangered Federal Legal Status: Threatened Description: Blue shiners grow to 10 cm (4 in) in length. Coloration is olive dorsally with silvery sides. A distinctive metallic blue-black lateral stripe runs from the gill covering to the caudal fin where it widens to form a spot at the base of the caudal fin. Scale edges above and below the lateral stripe are edged with melanophores to form a distinctive diamond shape. Mouth opens just below the snout tip and is slanted in profile. Breeding males develop intense yellow on all fins except the dorsal. Similar Species: Four other species of Cyprinella are known from the Conasauga River system: the Alabama shiner (C. callistia), the blacktail shiner (C. venusta) the introduced red shiner (C. lutrensis) and the tricolor shiner (C. trichroistia). The uniform width and intensity of the lateral stripe separates the blue shiner from all of these species. Habitat: The preferred habitat of blue shiners consists of small to medium streams that include rocky substrates. Fish are found in riffles and runs, as well as pools with moderate to swift current, over gravel to cobble or boulder substrate. Diet: Terrestrial insects captured from stream drift; also aquatic insects. Life History: Blue shiners have an extended spawning period from May to August. Eggs are deposited in silt free areas in rock crevices, or possibly crevices in woody debris, in habitats with moderate current. Life history studies on the blue shiner revealed that most individuals were sexually mature in the third summer of life and some were in their fourth. -

Fishes of Gus Engeling Wildlife Management Area
TEXAS PARKS AND WILDLIFE FISHES OF G U S E N G E L I N G WILDLIFE MANAGEMENT AREA A FIELD CHECKLIST “Act Natural” Visit a Wildlife Management Area at our Web site: http://www.tpwd.state.tx.us Cover: Illustration of Flathead Catfish by Rob Fleming. HABITAT DESCRIPTION he Gus Engeling Wildlife Management Area is located in the northwest corner of Anderson County, 20 miles Tnorthwest of Palestine, Texas, on U.S. Highway 287. The management area contains 10,941 acres of land owned by the Texas Parks and Wildlife Department. Most of the land was purchased in 1950 and 1951, with the addition of several smaller tracts through 1960. It was originally called the Derden Wildlife Management Area, but was later changed to the Engeling Wildlife Management Area in honor of Biologist Gus A. Engeling, who was killed by a poacher on the area in December 1951. The area is drained by Catfish Creek, an eastern tributary in the upper middle basin of the Trinity River. The topography is gently rolling to hilly, with a well-defined spring-fed drainage system of eight branches that empty into Catfish Creek. These streams normally flow year-round and the entire drainage system is flooded annually. Diverse wetland habitats include hardwood bottomland floodplain (2,000 acres), riparian corri dors (491 acres), marshes and swamps (356 acres), bogs (272 acres) and several beaver ponds of varying sizes and ages. The soils are mostly light colored, rapidly permeable sands on the upland, and moderately permeable, gray-brown, sandy loams in the bottomland along Catfish Creek. -
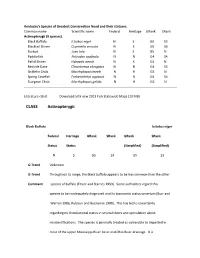
CLASS Actinopterygii
Kentucky's Species of Greatest Conservation Need and their statuses. Common name Scientific name Federal Heritage GRank SRank Actinopterygii (9 species). Black Buffalo Ictiobus niger N S G5 S3 Blacktail Shiner Cyprinella venusta N S G5 S3 Burbot Lota lota N S G5 N Paddlefish Polyodon spathula N N G4 S4 Pallid Shiner Hybopsis amnis N X G4 N Redside Dace Clinostomus elongatus N N G4 S3 Sicklefin Chub Macrhybopsis meeki N H G3 N Spring Cavefish Forbesichthys agassizii N N G4 S4 Sturgeon Chub Macrhybopsis gelida N H G3 N Literature cited Download all 9 new 2013 Fish Statewide Maps (10 MB) CLASS Actinopterygii Black Buffalo Ictiobus niger Federal Heritage GRank SRank GRank SRank Status Status (Simplified) (Simplified) N S G5 S3 G5 S3 G-Trend Unknown G-Trend Throughout its range, the black buffalo appears to be less common than the other Comment species of buffalo (Etnier and Starnes 1993). Some authorities regard this species to be inadequately diagnosed and its taxonomic status uncertain (Burr and Warren 1986, Robison and Buchanan 1988). This has led to uncertainty regarding its distributional status in several states and speculation about misidentifications. The species is generally treated as vulnerable to imperiled in most of the upper Mississippi River basin and Ohio River drainage. It is considered secure in only a few states in the middle and lower Mississippi River basin, although records in the Gulf Slope drainages in Texas and New Mexico are thought to potentially be based on misidentifications or introductions (Etnier and Starnes 1993, Natureserve 2008, Shute 1980). S-Trend Unknown S-Trend Burr and Warren (1986) regarded this species as sporadic and rare in rivers and Comment reservoirs in western Kentucky, and sporadic in the main channels of the Mississippi and Ohio rivers. -

TPWD Fish Identification
Fish Identification References • Hubbs, C., R.J. Edwards, and G.P. Garrett. 2008. An annotated checklist of the freshwater fishes of Texas, with keys to identification of species. Texas Academy of Science. Available from: http://www.texasacademyofscience.org/ • Page, L.M. and B.M. Burr. 2011. Peterson Field Guide to Freshwater Fishes, second edition. Houghton Mifflin Harcourt Trade and Reference Publishers. • Pflieger, W.L. 1997. The Fishes of Missouri. Missouri Department of Conservation. • Thomas, C., T.H. Bonner, and B.G. Whiteside. 2007. Freshwater Fishes of Texas. Texas A&M University Press, College Station, Texas. • Robison, H.W. and T.M. Buchanan. 1988. Fishes of Arkansas. The University of Arkansas Press, Fayetteville, Arkansas. Gars • Ganoid scales • Beaklike snout Alligator Gar: Teeth on upper jaw in two rows Gars Spotted Gar: One row of teeth on upper jaw, Snout short Longnose Gar: One row of teeth on upper jaw, Snout long and narrow Herrings • Lateral line absent • Keel along belly Gizzard Shad: Subterminal mouth, blunt snout, large spot Threadfin Shad: Terminal mouth, pointed snout, small spot Minnows Campostoma • Cartilaginous ridge on lower jaw • Intestine long, wound around air bladder Chad Thomas Cyprinella • Typically deep-bodied minnows • Dorsal fin with pigment between rays Red Shiner: Dark shoulder patch, chin bar Blacktail Shiner: Distinct caudal spot Cyprinella Notemigonus Golden Shiner: Lateral line greatly decurved Macrhybopsis Chad Thomas Chubs: Maxillary barbels present, most with speckling Differentiate by location -

Scoring Criteria for the Index of Biotic
Part III: Scoring Criteria for the Index of Biotic Integrity to Monitor Fish Communities in Wadeable Streams in the Apalachicola and Atlantic Slope Drainage Basins of the Southeastern Plains Ecoregion of Georgia Georgia Department of Natural Resources Wildlife Resources Division Fisheries Management Section 2020 Table of Contents Introduction………………………………………………………………… ………Pg. 1 Map of Southeastern Plains Ecoregion………………………………..…………… Pg. 3 Table 1. State Listed Fish in the Southeastern Plains Ecoregion………………….. Pg. 4 Table 2. IBI Metrics and Scoring Criteria………………………………………….Pg. 5 References………………………………………………….. ………………………Pg. 7 Appendix 1…………………………………………………………………………. Pg. 8 Apalachicola Basin Group (ACF) MSR Graphs…………………………… Pg. 9 Atlantic Slope Basins Group (AS) MSR Graphs…………………………... Pg. 17 Southeastern Plains Ecoregion Fish List……………………………………Pg. 25 i Introduction The Southeastern Plains ecoregion (SEP) is the largest of the six Level III ecoregions found in Georgia (Part I, Figure 1). It covers most of the southern portion of Georgia, bordering the Piedmont ecoregion to the north and the Southern Coastal Plain ecoregion to the southeast. It includes all or portions of 80 counties (Figure 1), covering a land area of over 25,000 square miles (United States Census Bureau 2000). Major drainage basins found within the (SEP) include the Chattahoochee, Flint, Ocmulgee, Oconee, Altamaha, Ogeechee, Savannah, Satilla, Suwannee, and Ochlockonee. The biotic index developed by the GAWRD is based on Level III ecoregion delineations (Griffith et al. 2001). The metrics and scoring criteria were developed from biomonitoring samples collected in the Chattahoochee, Flint, Ocmulgee, Oconee, Altamaha, Ogeechee, and the Savannah Drainage Basins. Based on similarities in species richness and composition, these seven drainages were aligned into two groups: the Apalachicola Drainage Basin (ACF), including the Chattahoochee and Flint drainage basins, and the Atlantic Slope Drainage Basin (AS), including the Altamaha, Ocmulgee, Oconee, Ogeechee, and Savannah Drainage Basins. -

Texas Freshwater Fish Species Checklist
TEXAS FRESHWATER FISH SPECIES CHECKLIST Order Common Name Genus species Location Collected LAMPREYS STATUS Chestnut Lamprey Ichthyomyzon castaneus Southern Brook Lamprey Ichthyomyzon gagei STURGEONS Shovelnose Sturgeon Scaphirhynchus platorynchus T PADDLEFISH Paddlefish Polyodon spathula T GARS Alligator Gar Atractosteus spatula Spotted Gar Lepisosteus oculatus Longnose Gar Lepisosteus osseus Shortnose Gar Lepisosteus platostomus BOWFINS Bowfin Amia calva FRESHWATER EELS American Eel Anguilla rostrata HERRINGS & SHADS Skipjack Herring Alosa chrysochloris Gizzard Shad Dorosoma cepedianum Threadfin Shad Dorosoma petenense MOONEYES Goldeye Hiodon alosoides Mooneye Hiodon tergisus TROUTS, SALMONS, ETC. * Rainbow Trout Onchorhynchus mykiss PIKES Grass Pickeral Esox americanus * Northern Pike Esox lucius Chain Pickeral Esox niger CHARACINS Mexican Tetra Astyanax mexicanus MINNOWS Central Stoneroller Campostoma anomalum * Goldfish Carassius auratus * Grass Carp Ctenopharyngodon idella Edwards Plateau Shiner Cyprinella lepida Red Shiner Cyprinella lutrensis Proserpine Shiner Cyprinella proserpina T Blacktail Shiner Cyprinella venusta Steelcolor Shiner Cyprinella whipplei * Common Carp Cyprinus carpio Devils River Minnow Dionda diaboli T Roundnose Minnow Dionda episcopa Speckled Chub Extrarius aestivalis Rio Grande Chub Gila pandora T E – Endangered Page 1 of 5 * Non-Native Species T – Threatened X – Extinct TEXAS FRESHWATER FISH SPECIES CHECKLIST Cypress Minnow Hybognathus hayi Mississippi Silvery Minnow Hybognathus nuchalis Plains Minnow Hybognathus -

Guide to the Fish of Turkey Creek
An Identification Guide to the Fishes of Turkey Creek Preserve, Jefferson County, Alabama Dr. R. Scot Duncan Department of Biology Birmingham-Southern College Box 549022 900 Arkadelphia Rd. Birmingham, AL 35254 USA Ph: (205) 226-4777 Email: [email protected] 2 Fishes collected at Turkey Creek, Jeff. Co, AL. List provided by Dr. Mike Howell of Samford University. Cyprinidae: Semotilus atromaculatus - creek chub Campostoma oligolepis - fine-scale stoneroller Notropis stilbius - silverstripe shiner Luxilus chrysocephalus - striped shiner Cyprinella venusta - blacktail shiner Fundulidae: Fundulus olivaceus - blackspotted topminnow Poeciliidae: Gambusia affinis - mosquitofish Catostomidae: Hypentelium etowanum - Alabama hogsucker Moxostoma duquesnei - black redhorse Moxostoma erythrurum - golden redhorse Percidae: Percina nigrofasciata - Blackbanded darter Percina kathae - Mobile logperch Etheostoma stigmaeum - Speckled darter Etheostoma jordani - Bluebreast darter (now Etheostoma douglasi) Etheostoma whipplei - Eastern Redfin darter *Etheostoma chermocki - Vermilion darter ^Etheostoma phytophilum - Rush darter *Etheostoma nuchale - Watercress darter (introduced by Dr. Howell during 1986 from Roebuck Springs) Centrarchidae: Lepomis cyanellus - Green sunfish Lepomis macrochirus - Bluegill Lepomis microlophus - Redear sunfish Micropterus salmoides - Largemouth bass Micropterus punctulatus - Spotted bass Micropterus coosae - Redeye bass Cottidae: Cottus carolinae – Banded Sculpin * listed by U. S. Fish & Wildlife Service as Endangered ^ a candidate species for Federal listing as endangered 3 Description of Fish Families: Use these descriptions and the pictures to determine to which family your specimen belongs. Then study the images and descriptions of species in that family to identify your specimen. Family: Cyprinidae (Minnows). These active small fish are often swimming at or near the surface. They often are found in shallow riffles with rocky or stone bottom. Many species of “shiners” are in this family. -

Pteronotropis Welaka), with NOTES on the OCCURRENCE of the IRONCOLOR SHINER (Notropis Chalybaeus), in ALABAMA, 2007
GEOLOGICAL SURVEY OF ALABAMA Berry H. (Nick) Tew State Geologist WATER INVESTIGATIONS PROGRAM Patrick E. O’Neil Director STATUS SURVEY OF THE BLUENOSE SHINER (Pteronotropis welaka), WITH NOTES ON THE OCCURRENCE OF THE IRONCOLOR SHINER (Notropis chalybaeus), IN ALABAMA, 2007 OPEN-FILE REPORT 0712 By J. Brett Smith, Thomas E. Shepard, Patrick E. O’Neil, E. Anne Wynn, and Cal C. Johnson Prepared in cooperation with Alabama Department of Conservation and Natural Resources Division of Wildlife and Freshwater Fisheries Tuscaloosa, Alabama 2008 CONTENTS Page Abstract..................................................................................................................................... 1 Introduction............................................................................................................................... 1 Acknowledgments..................................................................................................................... 5 Study area.................................................................................................................................. 5 Methods..................................................................................................................................... 6 Results and discussion .............................................................................................................. 6 Conclusions............................................................................................................................... 11 References cited.......................................................................................................................