Production of the a Subunit of Guanine Nucleotide-Binding Protein GO by Neuroendocrine Tumors1
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Endocrine Tumors of the Pancreas
Friday, November 4, 2005 8:30 - 10:30 a. m. Pancreatic Tumors, Session 2 Chairman: R. Jensen, Bethesda, MD, USA 9:00 - 9:30 a. m. Working Group Session Pathology and Genetics Group leaders: J.–Y. Scoazec, Lyon, France Questions to be answered: 12 Medicine and Clinical Pathology Group leader: K. Öberg, Uppsala, Sweden Questions to be answered: 17 Surgery Group leader: B. Niederle, Vienna, Austria Questions to be answered: 11 Imaging Group leaders: S. Pauwels, Brussels, Belgium; D.J. Kwekkeboom, Rotterdam, The Netherlands Questions to be answered: 4 Color Codes Pathology and Genetics Medicine and Clinical Pathology Surgery Imaging ENETS Guidelines Neuroendocrinology 2004;80:394–424 Endocrine Tumors of the Pancreas - gastrinoma Epidemiology The incidence of clinically detected tumours has been reported to be 4-12 per million inhabitants, which is much lower than what is reported from autopsy series (about 1%) (5,13). Clinicopathological staging (12, 14, 15) Well-differentiated tumours are the large majority of which the two largest fractions are insulinomas (about 40% of cases) and non-functioning tumours (30-35%). When confined to the pancreas, non-angioinvasive, <2 cm in size, with <2 mitoses per 10 high power field (HPF) and <2% Ki-67 proliferation index are classified as of benign behaviour (WHO group 1) and, with the notable exception of insulinomas, are non-functioning. Tumours confined to the pancreas but > 2 cm in size, with angioinvasion and /or perineural space invasion, or >2mitoses >2cm in size, >2 mitoses per 20 HPF or >2% Ki-67 proliferation index, either non-functioning or functioning (gastrinoma, insulinoma, glucagonoma, somastatinoma or with ectopic syndromes, such as Cushing’s syndrome (ectopic ACTH syndrome), hypercaliemia (PTHrpoma) or acromegaly (GHRHoma)) still belong to the (WHO group 1) but are classified as tumours with uncertain behaviour. -

C O N F E R E N C E 7 18 October 2017
Joint Pathology Center Veterinary Pathology Services WEDNESDAY SLIDE CONFERENCE 2017-2018 C o n f e r e n c e 7 18 October 2017 CASE I: F1753191 (JPC 4101076). veterinarian revealed a regenerative anemia, stress leukogram and hypoproteinemia Signalment: 9-year-old, female intact, Rock characterized by hypoalbuminemia and the Alpine goat, Capra aegagrus hircus, goat was treated with ivermectin. caprine. Bloodwork at CSU revealed hyperglycemia and elevated creatinine, creatine kinase and History: A 9-year-old, female intact Rock aspartate aminotransferase levels. A fecal Alpine goat presented to Colorado State floatation revealed heavy loads of coccidia, University Veterinary Teaching Hospital strongyles and Trichuris spp. During a nine two months prior to necropsy with a three- day hospitalization, the doe was treated with day history of hyporexia and lethargy which intravenous fluids, kaopectate, thiamine, had progressed to lateral recumbency and fenbendazole, sulfadimethoxine, oxy- complete anorexia. The referring tetracycline and multiple blood transfusions. veterinarian had previously diagnosed the After significant improvement of her clinical doe with louse infestation, endoparasites and signs and bloodwork, including partial a heart murmur. Bloodwork by the referring resolution of the dermatitis, the doe was Haired skin goat. The skin was dry, alopecia, and covered with hyperkeratotic crusts and ulcers. (Photo courtesy of: Colorado State University, Microbiology, Immunology, and Pathology Department, College of Veterinary Medicine and Biomedical Sciences, http://csucvmbs.colostate.edu/academics/mip/Pages/default.aspx) 1 discharged. exfoliating epithelial crusts which were often tangled within scant remaining hairs. Two months later, the goat presented with a This lesion most severely affected the skin one month history of progressive scaling and over the epaxials, the ventral abdomen and ulceration over the withers, dew claws, and teats, coronary bands and dew claws. -

Aichi Prefecture
Coordinates: 35°10′48.68″N 136°54′48.63″E Aichi Prefecture 愛 知 県 Aichi Prefecture ( Aichi-ken) is a prefecture of Aichi Prefecture Japan located in the Chūbu region.[1] The region of Aichi is 愛知県 also known as the Tōkai region. The capital is Nagoya. It is the focus of the Chūkyō metropolitan area.[2] Prefecture Japanese transcription(s) • Japanese 愛知県 Contents • Rōmaji Aichi-ken History Etymology Geography Cities Towns and villages Flag Symbol Mergers Economy International relations Sister Autonomous Administrative division Demographics Population by age (2001) Transport Rail People movers and tramways Road Airports Ports Education Universities Senior high schools Coordinates: 35°10′48.68″N Sports 136°54′48.63″E Baseball Soccer Country Japan Basketball Region Chūbu (Tōkai) Volleyball Island Honshu Rugby Futsal Capital Nagoya Football Government Tourism • Governor Hideaki Ōmura (since Festival and events February 2011) Notes Area References • Total 5,153.81 km2 External links (1,989.90 sq mi) Area rank 28th Population (May 1, 2016) History • Total 7,498,485 • Rank 4th • Density 1,454.94/km2 Originally, the region was divided into the two provinces of (3,768.3/sq mi) Owari and Mikawa.[3] After the Meiji Restoration, Owari and ISO 3166 JP-23 Mikawa were united into a single entity. In 187 1, after the code abolition of the han system, Owari, with the exception of Districts 7 the Chita Peninsula, was established as Nagoya Prefecture, Municipalities 54 while Mikawa combined with the Chita Peninsula and Flower Kakitsubata formed Nukata Prefecture. Nagoya Prefecture was renamed (Iris laevigata) to Aichi Prefecture in April 187 2, and was united with Tree Hananoki Nukata Prefecture on November 27 of the same year. -

Ohkagakuen University Japan Study Program (English) the Pull of Ohkagakuen University
Ohkagakuen University Japan Study Program (English) The Pull of Ohkagakuen University ♥ School customs to value the tradition as a women’s university ♥ Careful guidance based on the motto: ‘Be kind in education.’ ♥ Strengthened curriculum in which students can learn a wide range of knowledge of the world as well as that of contemporary Japanese society. ♥ The existence of a Course where students can learn a lot about tourism ♥ Enriched fieldwork opportunity in collaboration with the local government Contents ♥ Excellent environment to improve ♠ The Pull of Ohkagakuen University 2 Japanese and English ♠ Message from the Dean of the SLADE 3 ♠ Profile of Ohkagakuen University 4 ♥ Nationally well-known campus life ♠ Introduction of the campus facilities 5 where students play their roll ♠ Curriculum 6 independently ♠ Annual Events of the School of Liberal Arts 7 ♥ Easy access to main cities such as Tokyo ♠ Accommodation 8 and Osaka ♠ Living Expenses 9 ♠ Q & A 10 ♥ A good site for a school, adjacent to a big ♠ Access Information 11 city, still rich in nature ♠ Contact Details 12 OHKA 2 Message from the Dean of the SLADE The School of Liberal Arts, Department of English (SLADE) at Ohkagakuen University, welcomes international students from the bottom of its heart. The students of the SLADE have the experience of learning English abroad ten months after entering the university. They, therefore, are open-minded toward foreign people and cultures. Half of the faculty members are foreigners; their mother tongues being English, Korean and Chinese. All the Japanese staff has an experience of studying or doing research overseas. Still more, there is a Korean staff member in the office. -

Rising Incidence of Neuroendocrine Tumors
Rising Incidence of Neuroendocrine Tumors Dasari V, Yao J, et al. JAMA Oncology 2017 S L I D E 1 Overview Pancreatic Neuroendocrine Tumors • Tumors which arise from endocrine cells of the pancreas • 5.6 cases per million – 3% of pancreatic tumors • Median age at diagnosis 60 years • More indolent course compared to adenocarcinoma – 10-year overall survival 40% • Usually sporadic but can be associated with hereditary syndromes – Core genetic pathways altered in sporadic cases • DNA damage repair (MUTYH) Chromatin remodeling (MEN1) • Telomere maintenance (MEN1, DAXX, ATRX) mTOR signaling – Hereditary: 17% of patients with germline mutation Li X, Wang C, et al. Cancer Med 2018 Scarpa A, Grimond S, et al. NatureS L I2017 D E 2 Pathology Classification European American Joint World Health Organization Neuroendocrine Committee on Cancer (WHO) Tumor Society (AJCC) (ENETS) Grade Ki-67 Mitotic rt TNM TNM T1: limit to pancreas, <2 cm T1: limit to pancreas, ≦2 cm T2: limit to pancreas, 2-4 cm T2: >limit to pancreas, 2 cm T3: limit to pancreas, >4 cm, T3: beyond pancreas, no celiac or Low ≤2% <2 invades duodenum, bile duct SMA T4: beyond pancreas, invasion involvement adjacent organs or vessels T4: involves celiac or SMA N0: node negative No: node negative Intermed 3-20% 2-20 N1: node positive N1: node positive M0: no metastases M0: no metastases High >20% >20 M1: metastases M1: metastases S L I D E 3 Classification Based on Functionality • Nonfunctioning tumors – No clinical symptoms (can still produce hormone) – Accounts for 40% of tumors – 60-85% -

Preclinical Cushing's Syndrome Resulting from Adrenal Black
Endocrine Journal 2007, 54 (4), 543–551 Preclinical Cushing’s Syndrome Resulting from Adrenal Black Adenoma Diagnosed with Diabetic Ketoacidosis TOSHIO KAHARA, CHIKASHI SETO*, AKIO UCHIYAMA**, DAISUKE USUDA, HIROSHI AKAHORI, EIJI TAJIKA*, ATSUO MIWA**, RIKA USUDA, TAKASHI SUZUKI*** AND HIRONOBU SASANO*** Department of Internal Medicine, Toyama Prefectural Central Hospital, 2-2-78 Nishinagae, Toyama 930-8550, Japan *Department of Urology, Toyama Prefectural Central Hospital, 2-2-78 Nishinagae, Toyama 930-8550, Japan **Department of Pathology, Toyama Prefectural Central Hospital, 2-2-78 Nishinagae, Toyama 930-8550, Japan ***Department of Pathology, Tohoku University School of Medicine, 2-1 Seiryo-machi, Aoba-ku, Sendai 980-8575, Japan Abstract. A right adrenal tumor was incidentally discovered on abdominal computed tomography performed on a 53- year-old Japanese man, who had been hospitalized with diabetic ketoacidosis. Normal values were obtained for adrenal hormones in the morning after an overnight fast and urinary cortisol excretion after treatment of diabetic ketoacidosis with insulin. However, overnight dexamethasone administration with 1 mg or 8 mg did not completely suppress serum cortisol levels. There were no remarkable physical findings related to Cushing’s syndrome. The patient was diagnosed as having preclinical Cushing’s syndrome (PCS). Histological examination of the adrenalectomy specimen demonstrated adrenal black adenoma. Blood glucose levels subsequently improved after adrenalectomy, and the patient never developed adrenal insufficiency after hydrocortisone withdrawal. The patient was treated with diet therapy alone, and maintained good glycemic control. However, the patient still showed a diabetic pattern in an oral glucose tolerance test. It seems that the existence of PCS in addition to the underlying type 2 diabetes mellitus contributed to aggravation of blood glucose levels. -

Lions Clubs International Club Membership Register Summary the Clubs and Membership Figures Reflect Changes As of September 2004
LIONS CLUBS INTERNATIONAL CLUB MEMBERSHIP REGISTER SUMMARY THE CLUBS AND MEMBERSHIP FIGURES REFLECT CHANGES AS OF SEPTEMBER 2004 CLUB CLUB LAST MMR FCL YR MEMBERSHI P CHANGES TOTAL DIST IDENT NBR CLUB NAME STATUS RPT DATE OB NEW RENST TRANS DROPS NETCG MEMBERS 5498 024659 AGUI 334 A 4 09-2004 50 0 0 0 0 0 50 5498 024661 AMA 334 A 4 09-2004 45 1 0 0 0 1 46 5498 024662 ANJYO MINAMI 334 A 4 09-2004 93 0 0 0 0 0 93 5498 024663 ANJYO 334 A 4 09-2004 79 0 0 0 0 0 79 5498 024667 BISAI 334 A 4 09-2004 36 0 0 0 0 0 36 5498 024669 CHIRYU 334 A 4 09-2004 85 0 0 0 0 0 85 5498 024670 CHITA 334 A 4 09-2004 51 0 0 0 -2 -2 49 5498 024675 GAMAGORI 334 A 4 09-2004 61 2 0 0 -2 0 61 5498 024680 HANDA 334 A 4 09-2004 102 0 0 0 -1 -1 101 5498 024681 HEKINAN 334 A 4 09-2004 86 3 0 0 -1 2 88 5498 024685 ICHINOMIYA 334 A 4 09-2004 77 1 1 0 0 2 79 5498 024687 ICHINOMIYA BIHOKU 334 A 4 08-2004 9 0 0 0 0 0 9 5498 024688 INAZAWA 334 A 4 09-2004 72 0 0 0 0 0 72 5498 024689 ICHINOMIYA NAKA 334 A 4 09-2004 45 4 0 0 0 4 49 5498 024690 INUYAMA 334 A 4 09-2004 69 2 0 0 -2 0 69 5498 024691 ICHINOMIYA SOUTH 334 A 4 09-2004 65 1 0 0 -1 0 65 5498 024696 KARIYA KINUURA 334 A 4 09-2004 46 0 0 0 0 0 46 5498 024698 KANIE 334 A 4 09-2004 27 1 0 0 -2 -1 26 5498 024700 KARIYA 334 A 4 09-2004 69 1 0 0 0 1 70 5498 024701 KASUGAI 334 A 4 09-2004 53 0 0 0 0 0 53 5498 024703 KIRA 334 A 4 09-2004 51 1 0 0 0 1 52 5498 024707 KOMAKI 334 A 4 09-2004 62 5 0 0 0 5 67 5498 024709 KONAN 334 A 4 09-2004 109 2 1 0 -1 2 111 5498 024710 KOTA 334 A 4 09-2004 27 1 0 0 0 1 28 5498 024717 MIHAMA 334 -

The Glucagonoma Syndrome And
European Journal of Endocrinology (2004) 151 531–537 ISSN 0804-4643 REVIEW The glucagonoma syndrome and necrolytic migratory erythema: a clinical review Andre´ P van Beek, Ellen R M de Haas1, Willem A van Vloten2, Cees J M Lips3, Janine F M Roijers3 and Marijke R Canninga-van Dijk4 University Medical Center Groningen, Department of Endocrinology, PO Box 30.001, 9700 RB, Groningen, 1Erasmus MC - University Medical Center Rotterdam, Department of Dermatology, PO Box 1738, 3000 DR, Rotterdam, and Departments of 2Dermatology, 3Endocrinology and 4Pathology, University Medical Center Utrecht, PO Box 85.500, 3508 GA, Utrecht, The Netherlands (Correspondence should be addressed to A P van Beek; Email: [email protected]) Abstract The glucagonoma syndrome is a rare disease in which a typical skin disorder, necrolytic migratory erythema, is often one of the first presenting symptoms. Weight loss and diabetes mellitus are two other prevalent characteristics of this syndrome. Necrolytic migratory erythema belongs to the recently recognized family of deficiency dermatoses of which zinc deficiency, necrolytic acral erythema and pellagra are also members. It is typically characterized on skin biopsies by necrolysis of the upper epidermis with vacuolated keratinocytes. In persistent hyperglucagonemia, excessive stimulation of basic metabolic pathways results in diabetes mellitus at the expense of tissue glycogen stores, and muscle and fat mass. Multiple (essential) nutrient and vitamin B deficiencies develop, which contrib- ute to the dermatosis. In addition, glucagonomas may produce various other products, like pancreatic polypeptide, that add to the catabolic effects of glucagon. European Journal of Endocrinology 151 531–537 Introduction an appendectomy and at age 50 he was operated on because of bilateral inguinal herniae. -
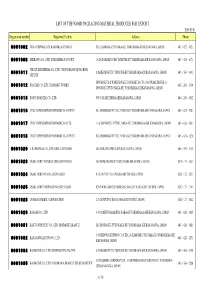
LIST of the WOOD PACKAGING MATERIAL PRODUCER for EXPORT 2005/03/01 Registration Number Registered Facility Address Phone
LIST OF THE WOOD PACKAGING MATERIAL PRODUCER FOR EXPORT 2005/03/01 Registration number Registered Facility Address Phone 0001002 ITOS CORPORATION KAMOME-JIGYOSHO 62-1 KAMOME-CHO NAKA-KU YOKOHAMA-SHI KANAGAWA, JAPAN 045‐622‐1421 0001008 ISHIKAWA CO., LTD. YOKOHAMA FACTORY 18-24 DAIKOKU-CHO TSURUMI-KU YOKOHAMA-SHI KANAGAWA, JAPAN 045‐521‐6171 THE IZUMI EXPRESS CO., LTD. TOKYO BRANCH, PACKING 0001011 8 DAIKOKU-FUTO TSURUMI-KU YOKOHAMA-SHI KANAGAWA, JAPAN 045‐504‐9431 CENTER HONMOKU B-2 WARE HOUSE, HONMOKU D-CFS 1 GO WARE HOUSE 3-1 0001012 INAGAKI CO., LTD. HONMOKU WORKS 045‐260‐1160 HONMOKU-FUTO NAKA-KU YOKOHAMA-SHI KANAGAWA, JAPAN 0001013 INOUE MOKUZAI CO., LTD. 895-3 SYAKE EBINA-SHI KANAGAWA, JAPAN 046‐236‐6512 0001016 UTOC CORPORATION HONMOKU B-1 OFFICE B-1, HONMOKU-FUTOU, NAKA-KU, YOKOHAMA-SHI, KANAGAWA, JAPAN 045‐621‐5781 0001017 UTOC CORPORATION HONMOKU D-5 CFS 1-16, HONMOKU-FUTOU, NAKA-KU, YOKOHAMA-SHI, KANAGAWA, JAPAN 045‐623‐1241 0001018 UTOC CORPORATION HONMOKU B-3 OFFICE B-3, HONMOKU-FUTOU, NAKA-KU, YOKOHAMA-SHI, KANAGAWA, JAPAN 045‐621‐6226 0001020 A.B. SHOHKAI CO., LTD. EBINA-JIGYOSHO 642 NAKANO EBINA-SHI KANAGAWA, JAPAN 046‐239‐0133 0001023 OSAKI CORP. TATEBAYASHI EIGYOUSHO 358 NOBE-MACHI TATEBAYASHI-SHI GUNMA, JAPAN 0276‐74‐6531 0001024 OSAKI CORP. OYAMA EIGYOUSHO 4-18-39 JYOUTOU OYAMA-SHI TOCHIGI, JAPAN 0285‐22‐1211 0001025 OSAKI CORP. NISHINASUNO EIGYOUSHO 429-9 NIKU-MACHI NISHINASUNO-CHO NASU-GUN TOCHIGI, JAPAN 0287‐37‐7161 0001028 OYAMA LUMBER CORPORATION 2-7-26 TENJIN-CHO OYAMA-SHI TOCHIGI, JAPAN 0285‐22‐0022 0001029 KAGAMI CO., LTD. -

Clinical Presentation and Diagnosis of Pancreatic Neuroendocrine Tumors
Clinical Presentation and Diagnosis of Pancreatic Neuroendocrine Tumors Carinne W. Anderson, MD*, Joseph J. Bennett, MD KEYWORDS Pancreatic neuroendocrine tumor Nonfunctional pancreatic neuroendocrine tumor Insulinoma Gastrinoma Glucagonoma VIPoma Somatostatinoma KEY POINTS Pancreatic neuroendocrine tumors are a rare group of neoplasms, most of which are nonfunctioning. Functional pancreatic neoplasms secrete hormones that produce unique clinical syndromes. The key management of these rare tumors is to first suspect the diagnosis; to do this, cli- nicians must be familiar with their clinical syndromes. Pancreatic neuroendocrine tumors (PNETs) are a rare group of neoplasms that arise from multipotent stem cells in the pancreatic ductal epithelium. Most PNETs are nonfunctioning, but they can secrete various hormones resulting in unique clinical syn- dromes. Clinicians must be aware of the diverse manifestations of this disease, as the key step to management of these rare tumors is to first suspect the diagnosis. In light of that, this article focuses on the clinical features of different PNETs. Surgical and medical management will not be discussed here, as they are addressed in other arti- cles in this issue. EPIDEMIOLOGY Classification PNETs are classified clinically as nonfunctional or functional, based on the properties of the hormones they secrete and their ability to produce a clinical syndrome. Nonfunctional PNETs (NF-PNETs) do not produce a clinical syndrome simply because they do not secrete hormones or because the hormones that are secreted do not The authors have nothing to disclose. Department of Surgery, Helen F. Graham Cancer Center, 4701 Ogletown-Stanton Road, S-4000, Newark, DE 19713, USA * Corresponding author. E-mail address: [email protected] Surg Oncol Clin N Am 25 (2016) 363–374 http://dx.doi.org/10.1016/j.soc.2015.12.003 surgonc.theclinics.com 1055-3207/16/$ – see front matter Ó 2016 Elsevier Inc. -
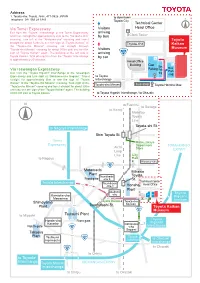
Address Via Tomei Expressway Via Isewangan Expressway to Nagoya
Address 1. Toyota-cho, Toyota, Aichi, 471-0826 JAPAN to downtown Telephone 081 565 29 3345 Toyota City Technical Center Via Tomei Expressway Visitors Head Office Exit from the “Toyota” Interchange of the Tomei Expressway arriving and travel straight for approximately 2km to the “Akebono-cho” by bus Clock Tower crossing. Turn left at the “Akebono-cho” crossing and travel Toyota straight for about 3.2km to see the sign of “Toyota Kaikan” at Toyota-cho Kaikan the “Toyota-cho Minami” crossing. Go straight through “Toyota-cho Minami” crossing for about 300m until you see the Visitors Museum sign of “Toyota Kaikan” again. The building at the left side is arriving Toyota Kaikan. Total driving time from the “Toyota” Interchange by car is approximately 20 minutes. Head Office Building Car Bus Parking Bus Via Isewangan Expressway Park Park Exit from the “Toyota Higashi” Interchange of the Isewangan -ing -ing Expressway and turn right at “Oshikomo-cho Gogami.” Travel to Toyota straight for approximately 3km to see the sign of “Toyota Interchange Kaikan” at the “Toyota-cho Minami” crossing. Turn right at the Toyota-cho Minami bus stop “Toyota-cho Minami” crossing and travel straight for about 300m Toyota Honsha Mae until you see the sign of the “Toyota Kaikan” again. The building at the left side is Toyota Kaikan. to Toyota Higashi Interchange / to Okazaki to Fushimi to Sanage to Kozoji Meitetsu Toyota Line Toyota-shi St. to Nagoya Interchange Shin Toyota St. Tomei Matsuzakaya Expressway Department TOKAI-KANJO Aichi Store EXPWY Loop Line City to Nagoya Hall Koromo-cho Motomachi Mikawa Plant Tsuchihashi Toyota Front gate -cho 5 St. -

Area Locality Address Description Operator Aichi Aisai 10-1
Area Locality Address Description Operator Aichi Aisai 10-1,Kitaishikicho McDonald's Saya Ustore MobilepointBB Aichi Aisai 2283-60,Syobatachobensaiten McDonald's Syobata PIAGO MobilepointBB Aichi Ama 2-158,Nishiki,Kaniecho McDonald's Kanie MobilepointBB Aichi Ama 26-1,Nagamaki,Oharucho McDonald's Oharu MobilepointBB Aichi Anjo 1-18-2 Mikawaanjocho Tokaido Shinkansen Mikawa-Anjo Station NTT Communications Aichi Anjo 16-5 Fukamachi McDonald's FukamaPIAGO MobilepointBB Aichi Anjo 2-1-6 Mikawaanjohommachi Mikawa Anjo City Hotel NTT Communications Aichi Anjo 3-1-8 Sumiyoshicho McDonald's Anjiyoitoyokado MobilepointBB Aichi Anjo 3-5-22 Sumiyoshicho McDonald's Anjoandei MobilepointBB Aichi Anjo 36-2 Sakuraicho McDonald's Anjosakurai MobilepointBB Aichi Anjo 6-8 Hamatomicho McDonald's Anjokoronaworld MobilepointBB Aichi Anjo Yokoyamachiyohama Tekami62 McDonald's Anjo MobilepointBB Aichi Chiryu 128 Naka Nakamachi Chiryu Saintpia Hotel NTT Communications Aichi Chiryu 18-1,Nagashinochooyama McDonald's Chiryu Gyararie APITA MobilepointBB Aichi Chiryu Kamishigehara Higashi Hatsuchiyo 33-1 McDonald's 155Chiryu MobilepointBB Aichi Chita 1-1 Ichoden McDonald's Higashiura MobilepointBB Aichi Chita 1-1711 Shimizugaoka McDonald's Chitashimizugaoka MobilepointBB Aichi Chita 1-3 Aguiazaekimae McDonald's Agui MobilepointBB Aichi Chita 24-1 Tasaki McDonald's Taketoyo PIAGO MobilepointBB Aichi Chita 67?8,Ogawa,Higashiuracho McDonald's Higashiura JUSCO MobilepointBB Aichi Gamagoori 1-3,Kashimacho McDonald's Gamagoori CAINZ HOME MobilepointBB Aichi Gamagori 1-1,Yuihama,Takenoyacho