Notes on Atomic Structure 1. Introduction 2. Hydrogen Atoms And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions
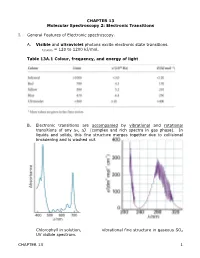
CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions I. General Features of Electronic spectroscopy. A. Visible and ultraviolet photons excite electronic state transitions. εphoton = 120 to 1200 kJ/mol. Table 13A.1 Colour, frequency, and energy of light B. Electronic transitions are accompanied by vibrational and rotational transitions of any Δν, ΔJ (complex and rich spectra in gas phase). In liquids and solids, this fine structure merges together due to collisional broadening and is washed out. Chlorophyll in solution, vibrational fine structure in gaseous SO2 UV visible spectrum. CHAPTER 13 1 C. Molecules need not have permanent dipole to undergo electronic transitions. All that is required is a redistribution of electronic and nuclear charge between the initial and final electronic state. Transition intensity ~ |µ|2 where µ is the magnitude of the transition dipole moment given by: ˆ d µ = ∫ ψfinalµψ initial τ where µˆ = −e ∑ri + e ∑Ziri € electrons nuclei r vector position of the particle i = D. Franck-Condon principle: Because nuclei are so much more massive and € sluggish than electrons, electronic transitions can happen much faster than the nuclei can respond. Electronic transitions occur vertically on energy diagram at right. (Hence the name vertical transition) Transition probability depends on vibrational wave function overlap (Franck-Condon factor). In this example: ν = 0 ν’ = 0 have small overlap ν = 0 ν’ = 2 have greatest overlap Now because the total molecular wavefunction can be approximately factored into 2 terms, electronic wavefunction and the nuclear position wavefunction (using Born-Oppenheimer approx.) the transition dipole can also be factored into 2 terms also: S µ = µelectronic i,f S d Franck Condon factor i,j i,f = ∫ ψf,vibr ψi,vibr τ = − CHAPTER 13 2 See derivation “Justification 14.2” II. -

Magnetism, Angular Momentum, and Spin
Chapter 19 Magnetism, Angular Momentum, and Spin P. J. Grandinetti Chem. 4300 P. J. Grandinetti Chapter 19: Magnetism, Angular Momentum, and Spin In 1820 Hans Christian Ørsted discovered that electric current produces a magnetic field that deflects compass needle from magnetic north, establishing first direct connection between fields of electricity and magnetism. P. J. Grandinetti Chapter 19: Magnetism, Angular Momentum, and Spin Biot-Savart Law Jean-Baptiste Biot and Félix Savart worked out that magnetic field, B⃗, produced at distance r away from section of wire of length dl carrying steady current I is 휇 I d⃗l × ⃗r dB⃗ = 0 Biot-Savart law 4휋 r3 Direction of magnetic field vector is given by “right-hand” rule: if you point thumb of your right hand along direction of current then your fingers will curl in direction of magnetic field. current P. J. Grandinetti Chapter 19: Magnetism, Angular Momentum, and Spin Microscopic Origins of Magnetism Shortly after Biot and Savart, Ampére suggested that magnetism in matter arises from a multitude of ring currents circulating at atomic and molecular scale. André-Marie Ampére 1775 - 1836 P. J. Grandinetti Chapter 19: Magnetism, Angular Momentum, and Spin Magnetic dipole moment from current loop Current flowing in flat loop of wire with area A will generate magnetic field magnetic which, at distance much larger than radius, r, appears identical to field dipole produced by point magnetic dipole with strength of radius 휇 = | ⃗휇| = I ⋅ A current Example What is magnetic dipole moment induced by e* in circular orbit of radius r with linear velocity v? * 휋 Solution: For e with linear velocity of v the time for one orbit is torbit = 2 r_v. -

Lecture B4 Vibrational Spectroscopy, Part 2 Quantum Mechanical SHO
Lecture B4 Vibrational Spectroscopy, Part 2 Quantum Mechanical SHO. WHEN we solve the Schrödinger equation, we always obtain two things: 1. a set of eigenstates, |ψn>. 2. a set of eigenstate energies, En QM predicts the existence of discrete, evenly spaced, vibrational energy levels for the SHO. n = 0,1,2,3... For the ground state (n=0), E = ½hν. Notes: This is called the zero point energy. Optical selection rule -- SHO can absorb or emit light with a ∆n = ±1 The IR absorption spectrum for a diatomic molecule, such as HCl: diatomic Optical selection rule 1 -- SHO can absorb or emit light with a ∆n = ±1 Optical selection rule 2 -- a change in molecular dipole moment (∆μ/∆x) must occur with the vibrational motion. (Note: μ here means dipole moment). If a more realistic Morse potential is used in the Schrödinger Equation, these energy levels get scrunched together... ΔE=hν ΔE<hν The vibrational spectroscopy of polyatomic molecules gets more interesting... For diatomic or linear molecules: 3N-5 modes For nonlinear molecules: 3N-6 modes N = number of atoms in molecule The vibrational spectroscopy of polyatomic molecules gets more interesting... Optical selection rule 1 -- SHO can absorb or emit light with a ∆n = ±1 Optical selection rule 2 -- a change in molecular dipole moment (∆μ/∆x) must occur with the vibrational motion of a mode. Consider H2O (a nonlinear molecule): 3N-6 = 3(3)-6 = 3 Optical selection rule 2 -- a change in molecular dipole moment (∆μ/∆x) must occur with the vibrational motion of a mode. Consider H2O (a nonlinear molecule): 3N-6 = 3(3)-6 = 3 All bands are observed in the IR spectrum. -

New Measurements of the Electron Magnetic Moment and the Fine Structure Constant
S´eminaire Poincar´eXI (2007) 109 – 146 S´eminaire Poincar´e Probing a Single Isolated Electron: New Measurements of the Electron Magnetic Moment and the Fine Structure Constant Gerald Gabrielse Leverett Professor of Physics Harvard University Cambridge, MA 02138 1 Introduction For these measurements one electron is suspended for months at a time within a cylindrical Penning trap [1], a device that was invented long ago just for this purpose. The cylindrical Penning trap provides an electrostatic quadrupole potential for trapping and detecting a single electron [2]. At the same time, it provides a right, circular microwave cavity that controls the radiation field and density of states for the electron’s cyclotron motion [3]. Quantum jumps between Fock states of the one-electron cyclotron oscillator reveal the quantum limit of a cyclotron [4]. With a surrounding cavity inhibiting synchrotron radiation 140-fold, the jumps show as long as a 13 s Fock state lifetime, and a cyclotron in thermal equilibrium with 1.6 to 4.2 K blackbody photons. These disappear by 80 mK, a temperature 50 times lower than previously achieved with an isolated elementary particle. The cyclotron stays in its ground state until a resonant photon is injected. A quantum cyclotron offers a new route to measuring the electron magnetic moment and the fine structure constant. The use of electronic feedback is a key element in working with the one-electron quantum cyclotron. A one-electron oscillator is cooled from 5.2 K to 850 mK us- ing electronic feedback [5]. Novel quantum jump thermometry reveals a Boltzmann distribution of oscillator energies and directly measures the corresponding temper- ature. -

The G Factor of Proton
The g factor of proton Savely G. Karshenboim a,b and Vladimir G. Ivanov c,a aD. I. Mendeleev Institute for Metrology (VNIIM), St. Petersburg 198005, Russia bMax-Planck-Institut f¨ur Quantenoptik, 85748 Garching, Germany cPulkovo Observatory, 196140, St. Petersburg, Russia Abstract We consider higher order corrections to the g factor of a bound proton in hydrogen atom and their consequences for a magnetic moment of free and bound proton and deuteron as well as some other objects. Key words: Nuclear magnetic moments, Quantum electrodynamics, g factor, Two-body atoms PACS: 12.20.Ds, 14.20.Dh, 31.30.Jv, 32.10.Dk Investigation of electromagnetic properties of particles and nuclei provides important information on fundamental constants. In addition, one can also learn about interactions of bound particles within atoms and interactions of atomic (molecular) composites with the media where the atom (molecule) is located. Since the magnetic interaction is weak, it can be used as a probe to arXiv:hep-ph/0306015v1 2 Jun 2003 learn about atomic and molecular composites without destroying the atom or molecule. In particular, an important quantity to study is a magnetic moment for either a bare nucleus or a nucleus surrounded by electrons. The Hamiltonian for the interaction of a magnetic moment µ with a homoge- neous magnetic field B has a well known form Hmagn = −µ · B , (1) which corresponds to a spin precession frequency µ hν = B , (2) spin I Email address: [email protected] (Savely G. Karshenboim). Preprint submitted to Elsevier Science 1 February 2008 where I is the related spin equal to either 1/2 or 1 for particles and nuclei under consideration in this paper. -

Lecture 17 Highlights
Lecture 23 Highlights The phenomenon of Rabi oscillations is very different from our everyday experience of how macroscopic objects (i.e. those made up of many atoms) absorb electromagnetic radiation. When illuminated with light (like French fries at McDonalds) objects tend to steadily absorb the light and heat up to a steady state temperature, showing no signs of oscillation with time. We did a calculation of the transition probability of an atom illuminated with a broad spectrum of light and found that the probability increased linearly with time, leading to a constant transition rate. This difference comes about because the many absorption processes at different frequencies produce a ‘smeared-out’ response that destroys the coherent Rabi oscillations and results in a classical incoherent absorption. Selection Rules All of the transition probabilities are proportional to “dipole matrix elements,” such as x jn above. These are similar to dipole moment calculations for a charge distribution in classical physics, except that they involve the charge distribution in two different states, connected by the dipole operator for the transition. In many cases these integrals are zero because of symmetries of the associated wavefunctions. This gives rise to selection rules for possible transitions under the dipole approximation (atom size much smaller than the electromagnetic wave wavelength). For example, consider the matrix element z between states of the n,l , m ; n ', l ', m ' Hydrogen atom labeled by the quantum numbers n,,l m and n',','l m : z = ψ()()r z ψ r d3 r n,l , m ; n ', l ', m ' ∫∫∫ n,,l m n',l ', m ' To make progress, first consider just the φ integral. -

Introduction to Differential Equations
Quantum Dynamics – Quick View Concepts of primary interest: The Time-Dependent Schrödinger Equation Probability Density and Mixed States Selection Rules Transition Rates: The Golden Rule Sample Problem Discussions: Tools of the Trade Appendix: Classical E-M Radiation POSSIBLE ADDITIONS: After qualitative section, do the two state system, and then first and second order transitions (follow Fitzpatrick). Chain together the dipole rules to get l = 2,0,-2 and m = -2, -2, … , 2. ??Where do we get magnetic rules? Look at the canonical momentum and the Asquared term. Schrödinger, Erwin (1887-1961) Austrian physicist who invented wave mechanics in 1926. Wave mechanics was a formulation of quantum mechanics independent of Heisenberg's matrix mechanics. Like matrix mechanics, wave mechanics mathematically described the behavior of electrons and atoms. The central equation of wave mechanics is now known as the Schrödinger equation. Solutions to the equation provide probability densities and energy levels of systems. The time-dependent form of the equation describes the dynamics of quantum systems. http://scienceworld.wolfram.com/biography/Schroedinger.html © 1996-2006 Eric W. Weisstein www-history.mcs.st-andrews.ac.uk/Biographies/Schrodinger.html Quantum Dynamics: A Qualitative Introduction Introductory quantum mechanics focuses on time-independent problems leaving Contact: [email protected] dynamics to be discussed in the second term. Energy eigenstates are characterized by probability density distributions that are time-independent (static). There are examples of time-dependent behavior that are by demonstrated by rather simple introductory problems. In the case of a particle in an infinite well with the range [ 0 < x < a], the mixed state below exhibits time-dependence. -

Class 35: Magnetic Moments and Intrinsic Spin
Class 35: Magnetic moments and intrinsic spin A small current loop produces a dipole magnetic field. The dipole moment, m, of the current loop is a vector with direction perpendicular to the plane of the loop and magnitude equal to the product of the area of the loop, A, and the current, I, i.e. m = AI . A magnetic dipole placed in a magnetic field, B, τ experiences a torque =m × B , which tends to align an initially stationary N dipole with the field, as shown in the figure on the right, where the dipole is represented as a bar magnetic with North and South poles. The work done by the torque in turning through a small angle dθ (> 0) is θ dW==−τθ dmBsin θθ d = mB d ( cos θ ) . (35.1) S Because the work done is equal to the change in kinetic energy, by applying conservation of mechanical energy, we see that the potential energy of a dipole in a magnetic field is V =−mBcosθ =−⋅ mB , (35.2) where the zero point is taken to be when the direction of the dipole is orthogonal to the magnetic field. The minimum potential occurs when the dipole is aligned with the magnetic field. An electron of mass me moving in a circle of radius r with speed v is equivalent to current loop where the current is the electron charge divided by the period of the motion. The magnetic moment has magnitude π r2 e1 1 e m = =rve = L , (35.3) 2π r v 2 2 m e where L is the magnitude of the angular momentum. -

Deuterium by Julia K
Progress Towards High Precision Measurements on Ultracold Metastable Hydrogen and Trapping Deuterium by Julia K. Steinberger Submitted to the Department of Physics in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY August 2004 - © Massachusetts Institute of Technology 2004. All rights reserved. Author .............. Department of Physics Augulst 26. 2004 Certifiedby......... .. .. .. .. .I- . .... Thom4. Greytak Prof or of Physics !-x. Thesis SuDervisor Certified by........................ Danil/Kleppner Lester Wolfe Professor of Physics Thesis Supervisor Accepted by... ...................... " . "- - .w . A. ....... MASSACHUSETTS INSTITUTE /Thom . Greytak OF TECHNOLOGY Chairman, Associate Department Head Vr Education SEP 14 2004 ARCHIVES LIBRARIES . *~~~~ Progress Towards High Precision Measurements on Ultracold Metastable Hydrogen and Trapping Deuterium by Julia K. Steinberger Submitted to the Department of Physics on August 26, 2004, in partial fulfillment of the requirements for the degree of Doctor of Philosophy Abstract Ultracold metastable trapped hydrogen can be used for precision measurements for comparison with QED calculations. In particular, Karshenboim and Ivanov [Eur. Phys. Jour. D 19, 13 (2002)] have proposed comparing the ground and first excited state hyperfine splittings of hydrogen as a high precision test of QED. An experiment to measure the 2S hyperfine splitting using a field-independent transition frequency in the 2S manifold of hydrogen is described. The relation between the transition frequency and the hyperfine splitting requires incorporating relativistic and bound state QED corrections to the electron and proton g-factors in the Breit-Rabi formula. Experimental methods for measuring the magnetic field of an ultracold hydrogen sample are developed for trap fields from 0 to 900 G. -

HJ Lipkin the Weizmann Institute of Science Rehovot, Israel
WHO UNDERSTANDS THE ZWEIG-IIZUKA RULE? H.J. Lipkin The Weizmann Institute of Science Rehovot, Israel Abstract: The theoretical basis of the Zweig-Iizuka Ru le is discussed with the emphasis on puzzles and paradoxes which remain open problems . Detailed analysis of ZI-violating transitions arising from successive ZI-allowed transitions shows that ideal mixing and additional degeneracies in the particle spectrum are required to enable cancellation of these higher order violations. A quantitative estimate of the observed ZI-violating f' + 2rr decay be second order transitions via the intermediate KK state agrees with experiment when only on-shell contributions are included . This suggests that ZI-violations for old particles are determined completely by on-shell contributions and cannot be used as input to estimate ZI-violation for new particle decays where no on-shell intermediate states exist . 327 INTRODUCTION - SOME BASIC QUESTIONS 1 The Zweig-Iizuka rule has entered the folklore of particle physics without any clear theoretical understanding or justification. At the present time nobody really understands it, and anyone who claims to should not be believed. Investigating the ZI rule for the old particles raises many interesting questions which may lead to a better understanding of strong interactions as well as giving additional insight into the experimentally observed suppression of new particle decays attributed to the ZI rule. This talk follows an iconoclastic approach emphasizing embarrassing questions with no simple answers which might lead to fruitful lines of investigation. Both theoretical and experimental questions were presented in the talk. However, the experimental side is covered in a recent paper2 and is not duplicated here. -

The Possible Explanation for the Mysterious Hot Solar Corona
Solar Physics DOI: 10.1007/•••••-•••-•••-••••-• A possible explanation for the mysterious hot solar corona Anil Raghav1 c Springer •••• Abstract The coronal heating has remained a puzzle for decades since its observation in 1940. Several space missions have been planned to resolve this conundrum in the past and many more intend to target this issue in the future. The unfolding of this issue will not only advance the fundamentals of astro- physical science but also promise an improvement in space weather prediction. Its origin has been debated without complete convergence; the acoustic waves, magneto-hydrodynamic waves, and micro/nano-flares are the strongest candi- dates so far to explain the mystery of coronal heating. However, none of these processes significantly justifies the million-degree temperature of the solar corona and the problem remains unsolved to date. Here, we propose a new physical mechanism to explain the observed heating of the solar corona. The statistical energy created during the interaction of the spin magnetic moment of the plasma particles with the turbulent and continuously evolving coronal magnetic field could substantiate the observed million-degree coronal temperature. Keywords: Corona, Magnetic fields The corona is the outermost layer of the Sun’s atmosphere, which can be observed as the thin white glow during a total solar eclipse (Saito & Tandberg- Hanssen, 1973). The spectroscopic measurements in 1940 discovered that the temperature of the corona is up to million degrees (Edl´en, 1937; Grotian, 1939; Lyot, 1939; Edl´en, 1945). Even eight decades after this discovery, here is still no concrete theory that explains this shooting up of temperature. -

Fundamental Physical Constants — Complete Listing Relative Std
From: http://physics.nist.gov/constants Fundamental Physical Constants — Complete Listing Relative std. Quantity Symbol Value Unit uncert. ur UNIVERSAL 1 speed of light in vacuum c; c0 299 792 458 m s− (exact) 7 2 magnetic constant µ0 4π 10− NA− × 7 2 = 12:566 370 614::: 10− NA− (exact) 2 × 12 1 electric constant 1/µ0c "0 8:854 187 817::: 10− F m− (exact) characteristic impedance × of vacuum µ / = µ c Z 376:730 313 461::: Ω (exact) p 0 0 0 0 Newtonian constant 11 3 1 2 3 of gravitation G 6:673(10) 10− m kg− s− 1:5 10− × 39 2 2 × 3 G=¯hc 6:707(10) 10− (GeV=c )− 1:5 10− × 34 × 8 Planck constant h 6:626 068 76(52) 10− J s 7:8 10− × 15 × 8 in eV s 4:135 667 27(16) 10− eV s 3:9 10− × 34 × 8 h=2π ¯h 1:054 571 596(82) 10− J s 7:8 10− × 16 × 8 in eV s 6:582 118 89(26) 10− eV s 3:9 10− × × 1=2 8 4 Planck mass (¯hc=G) mP 2:1767(16) 10− kg 7:5 10− 3 1=2 × 35 × 4 Planck length ¯h=mPc = (¯hG=c ) lP 1:6160(12) 10− m 7:5 10− 5 1=2 × 44 × 4 Planck time lP=c = (¯hG=c ) tP 5:3906(40) 10− s 7:5 10− × × ELECTROMAGNETIC 19 8 elementary charge e 1:602 176 462(63) 10− C 3:9 10− × 14 1 × 8 e=h 2:417 989 491(95) 10 AJ− 3:9 10− × × 15 8 magnetic flux quantum h=2e Φ0 2:067 833 636(81) 10− Wb 3:9 10− 2 × 5 × 9 conductance quantum 2e =h G0 7:748 091 696(28) 10− S 3:7 10− 1 × × 9 inverse of conductance quantum G0− 12 906:403 786(47) Ω 3:7 10− a 9 1 × 8 Josephson constant 2e=h KJ 483 597:898(19) 10 Hz V− 3:9 10− von Klitzing constantb × × 2 9 h=e = µ0c=2α RK 25 812:807 572(95) Ω 3:7 10− × 26 1 8 Bohr magneton e¯h=2me µB 927:400 899(37) 10− JT− 4:0 10− 1 × 5 1 × 9 in