Lechâtelier's Principle and the Solubility of Carbon Dioxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bromo Cresol Green Indicator I002
Bromo Cresol Green Indicator I002 Bromocresol green (BCG) indicator is used as a pH indicator in applications such as growth mediums for microorganisms and titrations. Composition** Ingredients Bromocresol green sodium salt 0.04 gm Disilled water 100.0ml **Formula adjusted, standardized to suit performance parameters Principle And Interpretation Bromocresol green (BCG) is a dye of the triphenylmethane family (triarylmethane dyes). The compound is synthesized by bromination of cresol purple (m-cresolsulfonphthalein). In aqueous solution, Bromocresol green will ionize to give the monoanionic form (yellow), that further deprotonates at higher pH to give the dianionic form (blue), which is stabilized by resonance. The Dissociation constant (pKa) of this reaction is 4.8. It becomes yellow at acidic pH level (pH 3.8) and Blue - green at from pH 5.4 . Quality Control Appearance Green coloured solution. Clarity Clear without any particles. Reaction At pH 3.8, the indicator turns yellow and at pH 5.4, the indicator is blue-green. Storage and Shelf Life Store between 10- 30°C. Use before expiry date on the label. Reference 1.Fred Senese. "Acid-Base Indicators". Frostburg State University Dept. of Chemistry. 2.Chemistry infolab reagents and resources ; The preparation of titration indicators; Dhanal De Lloyd,chem.Dept Revision : 1 / 2015 Disclaimer : User must ensure suitability of the product(s) in their application prior to use. Products conform solely to the information contained in this and other related HiMedia™ publications. The information contained in this publication is based on our research and development work and is to the best of our knowledge true and accurate. -

Bromocresol Green
Catalog Number: 101119, 152502, 152503, 195079, 806346, 806348 Bromocresol Green Structure: (sodium salt) Free Acid Sodium Salt Molecular Formula: C21H14Br4O5S C21H13Br4O5SNa Molecular Weight: 698.0 720 CAS # 76-60-8 62625-32-5 Synonyms: 3',3'',5',5''-Tetrabromo-m-cresolsulfonephthalein; Bromcresol Green Physical Description: Yellow to tan powder (free acid); Reddish-brown to Greenish-black crystalline powder (sodium salt) Solubility: Free Acid: Soluble in water (6 mg/ml), ethanol (40 mg/ml) or methyl cellosolve (200 mg/ml) Sodium Salt: Soluble in water (40 mg/ml) or ethanol (60 mg/ml). Description: Tracking dye for DNA agarose electrophoresis. lmax = 617 (400) nm in water. Also used as a pH indicator (3.8 to 5.4 : yellow to blue-green). Bromocresol green binds quantitatively with human albumin1 forming an intense blue-green complex with an absorbance maximum at 628 nm. The intensity of the color produced is directly proportional to the albumin concentration in the sample. Availability: Catalog Number Description Size 101119 Bromocresol Green 1 g 5 g 25 g 152502 Bromocresol Green, ACS Grade 1 g 5 g 25 g 152503 Bromocresol Green, sodium salt, ACS 1 gm Grade 5 gm 25 gm 195079 Bromocresol Green, sodium salt 1 gm 5 gm 10 gm 25 gm 806346 Bromocresol Green, sodium salt 5 gm 806348 10 gm References: 1. Kessler, M.A., et al., Anal. Biochem., v. 248, 180 (1997). 2. "Interaction of bromocresol green with different serum albumins studied by fluorescence quenching." Biochem. Mol. Biol. Int., v. 43:1, 1-8 (1997). 3. "Albumin standards and the measurement of serum albumin with bromocresol green. -

Application of Bromocresol Green and Bromothymol Blue for the Extractive Spectrophotometric Determination of Anti-Hypertensive Drugs
Journal of Applied Pharmaceutical Science Vol. 5 (07), pp. 122-129, July, 2015 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2015.50719 ISSN 2231-3354 Application of Bromocresol Green and Bromothymol Blue for the Extractive Spectrophotometric Determination of Anti-hypertensive Drugs Akram M. El-Didamony1, Sameh M. Hafeez2, Ahmed A. Saad1 1 Chemistry Department, Faculty of Science, Zagazig University, Zagazig 44519, Egypt. 2 Ismailia Chemical Laboratory, Forensic Medicine Authority, Justice Ministry, Egypt. ABSTRACT ARTICLE INFO Article history: Simple, selective and highly sensitive spectrophotometric methods are proposed for the rapid and accurate Received on: 10/04/2015 determination of anti-hypertensive drugs namely telmisartan (TEL), propranolol (PRO), bisoprolol (BIS) and Revised on: 02/05/2015 carvedilol (CRV) in tablets and biological fluids using bromocressol green (BCG) and bromothymol blue (BTB). Accepted on: 16/06/2015 The developed methods involve formation of stable yellow colored dichloromethane extractable ion-pair Available online: 27/07/2015 complexes of the amino derivative of four antihypertensive drugs such as TEL, PRO, BIS and CRV with two sulphonphthalein acid dyes, namely; BCG and BTB in acidic buffer. The effect of optimum conditions via pH on Key words: the ion-pair formation, reagent concentration, time and temperature and solvent was studied. The composition of Antihypertensive drugs, the ion-pairs was found 1: 1 by Job’s method. The established methods having high sensitivity and good Extraction spectrophotometry, selectivity could be applied to the determination of the studied drugs in pharmaceutical, urine and blood serum BCG and BTB dyes; Ion-pair samples with satisfactory results. -

Bromocresol Purple
Bromocresol Purple Catalog Number: 150518, 195080 Bromocresol Purple Structure :(free acid) Free Acid Sodium Salt Molecular Formula: C21H16Br2O5S C21H15Br2O5SNa Molecular Weight: 540.2 562.2 CAS # 115-40-2 62625-30-3 Synonym: 5',5''-Dibromo-o-cresolsulfonephthalein Physical Description: Reddish-brown crystalline powder (sodium salt); Orange to purplish-brown crystalline powder (free acid) pK: 6.3 Solubility: Free Acid: Soluble in water (20 mg/ml), ethanol (80 mg/ml) or methyl cellosolve (300 mg/ml). Sodium Salt: Soluble in water (80 mg/ml), ethanol (20 mg/ml) or methyl cellosolve (90 mg/ml). Description: Typically used as a pH indicator (5.2-6.8 : yellow to blue-purple). Bromocresol purple binds quantitatively with human serum1 albumin forming a stable complex with absorbance maximum at 600 nm. The intensity of the color produced is directly proportional to the albumin concentration in the sample. Availability: Catalog Number Description Size 195080 Bromocresol Purple 5 g 25 g 100 g 150518 Bromocresol Purple, Sodium Salt 5 g 25 g References: 1. Hill, P.G. and Wells, T.N.C., Ann. Clin. Biochem., v. 20, 264 (1983). 2. Orndorff, W.R. and Purdy, A.C., J. Am. Chem. Soc., v. 48, 2216 (1926). file:///C|/Users/Administrator/Desktop/新建文件夹/150518.htm[2013/3/4 13:44:01] Bromocresol Purple 3. "Reduction of reaction differences between human mercaptalbumin and human nonmercaptalbumin measured by the bromocresol purple method." Clin. Chim. Acta, v. 289:1-2, 69-78 (1999). 4. "A new photometric assay with bromocresol purple for testing in vitro antitrichomonal activity in aerobic environment." Arzneimittelforschung, v. -

CRC Handbook of Chemistry and Physics, 91Th Edition
ACID-BASE INDICATORS A. K. Covington The first part of this table lists some common acid-base indica- The color code is: tors in alphabetical order along with the approximate pH range(s) C = colorless A = amber B/G = blue-green at which a color change occurs. Following this is a table of the Pk = pink Y = yellow V = violet same indicators ordered by pH range, which includes the nature of R = red B = blue P = purple the color change, instructions on preparation of the indicator solu- O = orange tion, and the acid dissociation constant pK, when available. Reference Bishop, E., Ed., Indicators, Pergamon, Oxford, 1972. Indicator pH Range Indicator pH Range Alizarin 5.6-7.2; 11.0-12.4 Ethyl bis(2,4-dimethylphenyl)ethanoate 8.4-9.6 Alizarin Red S 4.6-6.0 Ethyl Orange 3.4-4.8 Alizarin Yellow R 10.1-12.0 Ethyl Red 4.0-5.8 Benzopurpurine 4B 2.2-4.2 Ethyl Violet 0.0-2.4 4,4’-Bis(2-amino-1- 5,5’-Indigodisulfonic acid, disodium salt 11.4-13.0 naphthylazo)-2,2’- Malachite Green 0.2-1.8 stilbenedisulfonic acid 3.0-4.0 Metacresol Purple 1.2-2.8; 7.4-9.0 4,4’-Bis(4-amino-1- Metanil Yellow 1.2-2.4 naphthylazo)-2,2’- Methyl Green 0.2-1.8 stilbenedisulfonic acid 8.0-9.0 Methyl Orange 3.2-4.4 Brilliant Yellow 6.6-7.8 Methyl Red 4.8-6.0 Bromocresol Green 3.8-5.4 Methyl Violet 0.0-1.6 Bromocresol Purple 5.2-6.8 p-Naphtholbenzein 8.2-10.0 Bromophenol Blue 3.0-4.6 Neutral Red 6.8-8.0 Bromothymol Blue 6.0-7.6 p-Nitrophenol 5.4-6.6 Chlorophenol Red 5.2-6.8 m-Nitrophenol 6.8-8.6 Clayton Yellow 12.2-13.2 Orange IV 1.4-2.8 Congo Red 3.0-5.0 Paramethyl Red 1.0-3.0 -

Kinetic and Thermodynamic Study of the Reaction Between Bromocresol Green with Sodium Hydroxide in an Aqueous Solution
JournalJournal of Chemical of Chemical Technology Technology and and Metallurgy, Metallurgy, 54, 54,1, 2019, 1, 2019 90-94 KINETIC AND THERMODYNAMIC STUDY OF THE REACTION BETWEEN BROMOCRESOL GREEN WITH SODIUM HYDROXIDE IN AN AQUEOUS SOLUTION Latona Dayo Felix1, Adejoro Ajibade2 1 Department of Chemical Sciences, Osun State University Received 15 November 2017 PMB 4494 Osogbo, Nigeria Accepted 06 March 2018 2 Department of Chemistry, University of Ibadan, Nigeria E-mail: [email protected] ABSTRACT The kinetics and the mechanism of the reaction of Bromocresol green and a hydroxyl ion in an aqueous solution is studied spectrophotometrically. The reaction is found of a first order in respect to both reactants. The Michaelis-Menten plot shows intermediate complex presence and reaction dependence on the solution ionic strength. The activation param- eters are evaluated. The negative ΔS‡ value obtained indicates an associative mechanism. Keywords: kinetics, thermodynamics, mechanism, Bromocresol green (BCG), sodium hydroxide and potassium nitrate. INTRODUCTION to emptying them into the water bodies as industrial ef- fluents is imminent in order to protect the aquatic organ- Bromocresol green belongs to the triphenylmethane isms [3]. Several methods of achieving this noble objec- dye family. It is an important industrial raw material tive are reported [4-7]. However, this research tends to in textile, leather, paper, printing, plastic and ceramic investigate the option of hydrolyzing Bromocresol green industries [1]. Bromocresol green is employed in col- in an alkaline medium with the view to ascertaining the orimetric detection technologies and for visualization of kinetics and the mechanism of the reaction, which has compounds of a functional group whose pKa is below hitherto not been reported in the literature. -
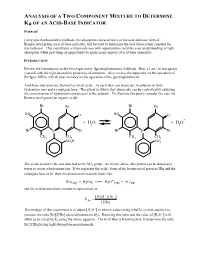
DETERMINATION of Ka of an ACID-BASE INDICATOR
ANALYSIS OF A TWO COMPONENT MIXTURE TO DETERMINE Ka OF AN ACID-BASE INDICATOR PURPOSE Using spectrophotometric methods, the absorption characteristics of the acid and base form of Bromocresol green, an acid-base indicator, will be used to determine the acid dissociation constant for this indicator. This experiment will provide you with opportunities to refine your understanding of light absorption while providing an opportunity to apply many aspects of acid-base chemistry. INTRODUCTION Review the Introduction to the first experiment “Spectrophotometric Methods: Beer’s Law” to reacquaint yourself with the light absorption properties of solutions. Also, review the Appendix on the operation of the Spec 20D to refresh your memory on the operation of the spectrophotometer. Acid-base indicators are themselves weak acids. As such they can dissociate in solution to form hydronium ions and a conjugate base. The extent to which they dissociate can be controlled by adjusting the concentration of hydronium ions present in the solution. To illustrate this point, consider the case for Bromocresol green (an organic acid): Br Br Br Br HO O HO O + + H2 O + H3 O Br C Br Br C Br CH3 CH3 CH3 CH3 - SO3 H SO3 The acidic proton is the one attached to the SO3 group. As shown above, this proton can be donated to water to create a hydronium ion. If we represent the acidic form of the bromocresol green as HIn and the conjugate base as In- then the dissociation reaction looks like: - HIn(aq) + H2O(l) <==> H3O+(aq) + In (aq) and the acid dissociation constant is represented as: [H O+ ][In− ] K = 3 In [HIn] + The strategy of this experiment is to adjust [H3O ] to known values using a buffer system and then to - + measure the ratio [In ]/[HIn] spectrophotometrically. -

Acid-Base Reactions in Organic Solvents. Behavior of Some
U . S. Department of Commerce Research Paper RPl900 National Bureau of Standards Volume 41, July 1948 Part of the Journal of Research of the National Bureau of Standards Acid-Base Reactions in Organic Solvents. Behavior of Some Halogenated Derivatives of Phenolsulfone phthalein with Different Classes of Organic Bases in Benzene By Marion Maclean Davis, Priscilla J. Schuhmann, and Mary Ellen Lovelace This paper, the second in a series co ncerning the use of indicator dyes to st udy t he re actions of organic acids a nd bases in o rganic solvents, deals with halogen derivatives of phenol sulfonepht halein. Spectrophotom etric data arc given for mixt ures of bromocresol gree n, bromophenol blu e, iodophenol blue, a nd tetrabromophenol blue wit h prima ry, seconda ry, and tertia ry aliphatic amines in benzene; and qualita tive data a re tabulated fo r chlorophenol blue, b romochl orophenol bllle, chlorophenol red , b romophe nol red , bromocresol purple, and brom othymol blue. Comparisons a re m ade of the p henolsulfon eph t halein s a nd t he bromo pht halein magentas. The st ru ctura l changes that accompa ny t he color p henomena a re discussed. Sugges t ions a re m ade regard in g t he use of the indicators in in ert solvents. I. Introduction occ urs during the second stage of neu tralization depends upon the nature of the base that is add ed. A previous article [ljl described in detail the It is differen t for primary, secondary , and ter tiary , preparation of the acidic indicators, bromo aliphatic amines. -

Determining the Acid Dissociation Constant for an Organic Dye
Determining the Acid Dissociation Constant for an Organic Dye One of the simplest ways to estimate a solution’s pH is to add a small amount of an organic dye and observe the solution’s color. For example, a solution of bromothymol blue—one of several sulfonophthalein dyes—is yellow when the pH is less than 6 and blue when the pH is greater than 8. Figure 1 shows the structure of bromothymol blue in its weak acid and weak base forms. When the pH of a solution of bromothymol blues is adjusted from 6 to 8, its color transitions from yellow to blue by passing through various shades of yellow-green, green, and green-blue, each of which correlates to within a few tenths of a pH unit. This relationship between the change in color and pH, is easy to appreciate from the basics of mixing color. Less obvious is the mathematical relationship between absorbance and pH A − AIn log = pKa − pH AHIn − A where A is the absorbance at a particular pH and AIn and AHIn are the absorbance values for the weak base and weak acid forms, respectively. Note that this equation suggests it is possible to determine a dye’s pKa by monitoring its absorbance as a function of pH. The purpose of this two-week, open-ended project is to design and carry out suitable experiments that can provide the pKa value for two organic dyes: bromocresol green and neutral red. Preparing for Lab Planning for this lab is critical to your success. Read the following paper as it provides useful information for determining the pKa values for organic dyes; a copy of the paper is in your group’s Dropbox folder. -

Impact of Impurities in Bromocresol Green Indicator Dye On
https://doi.org/10.5194/os-2019-126 Preprint. Discussion started: 9 January 2020 c Author(s) 2020. CC BY 4.0 License. Impact of impurities in bromocresol green indicator dye on spectrophotometric total alkalinity measurements Katharina Seelmann1, Martha Gledhill1, Steffen Aßmann2, and Arne Körtzinger1,3 1GEOMAR Helmholtz Centre for Ocean Research Kiel, Kiel, Germany 2Kongsberg Maritime Contros GmbH, Kiel, Germany 3Christian-Albrechts-Universität zu Kiel, Kiel, Germany Correspondence: Katharina Seelmann ([email protected]) Abstract. Due to its accurate and precise character, the spectrophotometric pH detection is a common technique applied in measurement methods for carbonate system parameters. However, impurities in the used pH indicator dyes can influence the measurements quality. The work described here focuses on influences from impurities in the pH indicator dye bromocresol green (BCG) on spectrophotometric seawater total alkalinity (AT) measurements. First, a high-performance liquid chromatog- 5 raphy (HPLC) purification method for BCG was developed. A subsequent analysis of BCG dye from four different vendors with this method revealed different types and quantities of impurities. After successful purification, AT measurements with purified and unpurified BCG were carried out using the novel autonomous analyzer CONTROS HydroFIAr TA. Long-term measurements in the laboratory revealed a direct influence of impurity types and quantities on the drift behavior of the analyzer. The purer the BCG, the smaller was the drift increment per measurement. Furthermore, we could show that a certain impurity 10 in some indicator dyes changed the drift pattern from linear to non-linear, which can impair the AT measurements during a long-term deployment of the system. -

University of Southampton Research Repository Eprints Soton
University of Southampton Research Repository ePrints Soton Copyright © and Moral Rights for this thesis are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given e.g. AUTHOR (year of submission) "Full thesis title", University of Southampton, name of the University School or Department, PhD Thesis, pagination http://eprints.soton.ac.uk UNIVERSITY OF SOUTHAMPTON An Automated Instrument for Measurement of Total Alkalinity in Seawater David Robert Owsianka Thesis for the degree of Doctor of Philosophy FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences 24 April 2014 UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences Doctor of Philosophy An Automated Instrument for Measurement of Total Alkalinity in Seawater by David Robert Owsianka Understanding the effects of increased CO2 uptake on the marine environment is a high priority for scientific study, as this leads to acidification. Precise means of measuring the degree of acidification, and doing so regularly over long time periods is a key requirement in separating natural from man‐made variation. This project examines new technologies for development of an instrument to measure one of the four main carbonate system variables, total alkalinity (TA), which is determined by acid/base titration. -

Experiment # 11: Spectroscopic Determination of Indicator Pka Ph Indicators May Be Defined As Highly Colored Bronsted-Lowry Acid-Base Conjugate Pairs
Experiment # 11: Spectroscopic determination of indicator pKa pH indicators may be defined as highly colored Bronsted-Lowry acid-base conjugate pairs. Used in low concentrations, these compounds signal pH changes within a specific range determined by the particular indicator in use. This color change range depends upon the relative acid strength (or pKa ) of the conjugate acid form of the indicator. In many indicator systems, both conjugate species are colored, and, within the pH transition range, the observed color is really a mixture of the colors of the two forms. The ratio of concentrations of the conjugate acid and base forms is controlled by the pH of the solution, as indicated by the same equation as that used for determining buffer pH’s, namely, the Henderson-Hasselbalch equation: ⎛⎞[conjugate base]⎛⎞ [Ind_ ] log⎜⎟= log⎜⎟ = pH - pKa ⎝⎠[conjugate acid]⎝⎠ [HInd ] where HInd and Ind ─ represent the acidic and anionic (or basic) forms of the indicator, respectively. The indicator concentration ratio is controlled by the pH of the solution, whereas the buffer concentration ratio controls the solution pH. At first glance, this statement seems contradictory. In reality, however, it is simply a matter of relative concentrations. The buffer components are present in high concentrations, so they control the pH of the buffer solution via the conventional acid-base reactions. On the other hand, the indicator species are present in low, even negligible, amounts relative to the other acid-base systems in the solution. In terms of visible absorption, however, the indicator species predominate over the buffer components (most of which are colorless). Procedures for determining solution pH using a short range of visual comparisons of indicator colors can usually distinguish 0.2 pH unit differences over a pH range of 2.0 units.